Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Photocatalyst converts fatty acids to diesel and jet-fuel molecules selectively
Method provides petroleum-free way to turn industrial biowaste into valuable commodity
by Mitch Jacoby
March 7, 2020
| A version of this story appeared in
Volume 98, Issue 9
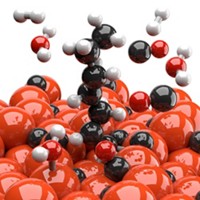
Industrial waste containing bioderived long-chain fatty acids could serve as sustainable feedstocks for diesel and jet fuels, which today are produced by refining petroleum sources. But several of the methods for removing oxygen from fatty acids to convert them to long-chain alkanes, the principal components of these fuels, require temperatures above 250 °C and high-pressure hydrogen, making them expensive and energy intensive. Decarboxylation methods, which convert fatty acids to alkanes by stripping carbon dioxide groups, run under milder conditions. But these methods tend to suffer from low selectivity: they generate a distribution of desirable and undesirable products. Now, Zhipeng Huang, Zhitong Zhao, and Feng Wang of the Dalian Institute of Chemical Physics and coworkers report that under mild conditions (30 °C and 0.2 MPa hydrogen) and in the presence of ultraviolet light, a Pt-TiO2 catalyst decarboxylates fatty acids selectively (Nat. Catal. 2020, DOI: 10.1038/s41929-020-0423-3). For example, the method converted pure stearic and linoleic acids to n-heptadecane in greater than 90% yield. In tests of crude soybean and tall-oil fatty acids, which are inedible by-products of soybean processing and the pulp industry, respectively, the method produced mixtures of long-chain alkanes at up to roughly 90% yield.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter