Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Photocatalyst flips common sugars to rare ones
Method can break and re-form a specific C–H bond, changing the molecule’s stereochemistry, without need for protecting groups
by Leigh Krietsch Boerner
January 16, 2020
| A version of this story appeared in
Volume 98, Issue 3
Rare sugars such as D-tagatose and D-allose are in demand as both chemical feedstocks and building blocks in the pharmaceutical industry. But they’re called rare because they don’t occur commonly in nature. Chemists would like to source these rare sugars from biomass, but it’s complicated to transform the common sugars in those natural sources into their rare counterparts.
Now, Alison Wendlandt and coworkers at the Massachusetts Institute of Technology have found a straightforward way to switch up the stereochemistries of common sugars, turning them into rare ones (Nature 2020, DOI: 10.1038/s41586-020-1937-1).
Converting between sugars usually involves isomerization, such as the aldose-ketose isomerization, but in some cases it requires epimerization, changing the stereochemistry of a single carbon center.
In the new work, Wendlandt and her team used a blue light–emitting diode, an amine, a photocatalyst, and a thiol to perform site-selective epimerization reactions at 63–88% yields, which are higher than those of enzymatic reactions or previous chemical methods.
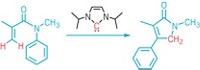
The reaction occurs through two steps in one reaction pot. First an amine radical formed by the photocatalyst pulls off a specific hydrogen atom from the sugar molecule, and then the cysteine thiol transfers a new H atom to the same carbon on the sugar but in a different stereochemical position (shown). The researchers can break a specific C–H bond on common sugars with this method. The basis for this selectivity is not fully understood, Wendlandt says. Previous chemical methods required chemists to add protecting groups on the C–H bonds that they were not targeting for epimerization. The new method is more direct and saves time and energy in synthesizing these rare sugars.
Many chemists avoid using rare sugars because they’re hard to access, but this chemistry may change that, says Adriaan Minnaard, an organometallic chemist from the University of Groningen. This work also opens a robust new synthetic platform that will help steer further research, says Núria López, a catalytic chemist at the Institute of Chemical Research of Catalonia.
CORRECTION
This story was updated on Jan. 17, 2019, to replace the third paragraph, which had an incorrect statement about the isomerization involved in transforming glucose into high-fructose corn syrup.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter