Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Pinning down the water-gas shift mechanism
Study identifies catalytically active sites and key reaction intermediates in classic industrial chemical process
by Mitch Jacoby
September 28, 2019
| A version of this story appeared in
Volume 97, Issue 38

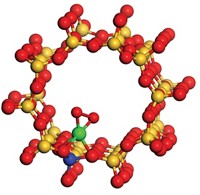
Industry has depended on the water-gas shift (WGS) reaction for more than a century. The WGS process, which combines carbon monoxide and water to form carbon dioxide and hydrogen, is a key component of industrial plants that use hydrogen to process hydrocarbons or to synthesize ammonia or methanol. The intimately related reverse process, RWGS, consumes CO2, a greenhouse gas, and generates CO, a valuable reagent. This chemistry has a long history, yet scientists continue to debate the nature of the catalytically active site and reaction pathway, key pieces of information needed for improving catalyst performance. Vassiliki-Alexandra “Vanda” Glezakou, János Szanyi, and coworkers at Pacific Northwest National Laboratory hope to end the debate. In a study combining spectroscopy, kinetics, and computations, the team analyzed the RWGS reaction driven by a palladium-alumina catalyst. They found that the key intermediate is a carboxylate species that bridges palladium and aluminum atoms (shown) and that a formate intermediate, proposed by other researchers, is a minor player in this chemistry (Nat. Catal. 2019, DOI: 10.1038/s41929-019-0343-2). The team determined that the catalytically active sites, which form only under reaction conditions, are hydroxides coupled to negatively charged palladium atoms at the Pd-Al2O3 interface.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter