Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Recycling plastic waste using a low-cost catalyst
Hydrogenation converts polyethylene and other common polyolefins to low-mass hydrocarbons
by Mitch Jacoby
December 6, 2022

An inexpensive catalyst can convert common commercial polymers to low molecular mass hydrocarbons under mild conditions, according to a new study. The advance may lead to cost-effective ways to produce liquid fuels and fine chemicals from plastic waste.
Researchers worldwide are developing methods for recycling plastics by chemically decomposing polymers into smaller molecular units and reusing them. Pyrolysis, for example, an approach backed by major chemical companies, converts plastics to pyrolysis oil, which can be used as a fuel or a feedstock to make other compounds. But this process is energy intensive—it often runs at 500 °C, and it produces a large mixture of products and carbonaceous waste.
Catalytic depolymerization can be more selective. And recent studies have shown that catalytic hydrogenolysis, which uses hydrogen to decompose plastics, is effective in breaking down polyethylene, polypropylene, and other common polyolefins, which tend to be fairly inert because of their strong carbon-carbon bonds. But hydrogenolysis generally uses platinum or other expensive catalysts, and it requires high temperatures and long reaction times.
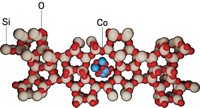
To get around those shortcomings, a team of researchers based at Northwestern University looked for a highly active earth-abundant catalyst that quickly drives hydrogenolysis of polyolefins under mild conditions. Led by Michael J. Bedzyk, Yosi Kratish, and Tobin J. Marks, the team reports that they came up with an organozirconium compound supported on sulfated alumina that does the job well (Nat. Commun. 2022, DOI: 10.1038/s41467-022-34707-6).
The team studied decomposition of several polymer samples, including polypropylene, polyethylene food packaging, polyethylene copolymers, and other types of polyethylene. In one test, they found that treating 1.5 g of polyethylene with 202 kPa (2 atm) of hydrogen at 200 °C in the presence of a small amount of the catalyst completely converted the polymer to liquid and gas products in just 45 minutes. Other samples also quickly turned into low-weight hydrocarbon products. The team’s experimental and computational investigations indicate that decomposition occurs via a C–C bond scission step rather than by way of σ-bond metathesis, which is common for other catalysts.
Colorado State University’s Eugene Chen says that one of the most intriguing aspects of this “exciting and insightful” work is that the proposed catalytic species responsible for this fast polyolefin deconstruction also represents the form of a polymerization catalyst that converts α-olefins to polyolefins. “It’s conceivable that this catalyst could be used for both polyolefin production and deconstruction simply by switching hydrogen on and off and adjusting the reaction temperature,” he says.
Susannah L. Scott of the University of California, Santa Barbara, says it’s remarkable that the active form of the catalyst appears to be air stable. This is important, she explains, because real plastic waste will have many different contaminants that the catalyst must tolerate. She notes, however, that the quantity of hydrogen used in this study may represent a challenge for a large-scale process. To convert all the world’s polyolefin waste in this way would require a significant fraction of current world hydrogen production, she says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter