Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Strained reagent opens up new cross-coupling reactivity
The twist on the Suzuki-Miyaura reaction offers a simple route to trisubstituted cyclobutanes
by Tien Nguyen
December 13, 2018
| A version of this story appeared in
Volume 96, Issue 49

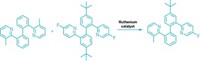
The Suzuki-Miyaura transformation is a classic way to form carbon-carbon bonds. In the reaction, a palladium catalyst typically couples two classes of molecules, aryl halides and aryl boronate complexes. Now researchers have taken a new approach to the traditional cross-coupling, with a method in which palladium reacts with the boronate compound not at the boron atom but at the neighboring carbon-carbon σ bond (Nat. Chem. 2018, DOI: 10.1038/s41557-018-0181-x). The result is a route to trisubstituted cyclobutanes valued by drug designers.
Led by the University of Bristol’s Varinder K. Aggarwal, the team accesses this novel mode of reactivity by using a strange-looking, bicyclobutyl sulfoxide reagent that exists as a bench-stable solid. Because of the strain placed on it, the cyclobutane’s middle carbon-carbon bond is weakened so it doesn’t act like a normal sigma bond, Aggarwal explains. At first, the reagent “seemed so unusual and esoteric,” he says, “but it’s actually easy to make and it does all this beautiful chemistry.”
Starting from the bicyclobutane precursor, the researchers could form bicyclobutyl boronate intermediates that could react with a variety of coupling partners to form trisubstituted cyclobutane products (example shown). These products contain substitution patterns similar to those made via [2+2] cycloaddition reactions, without that transformations’ requirement that one partner contain an electron-withdrawing substituent.
Boston College’s James P. Morken, whose group has expanded the Suzuki-Miyaura reaction to work with vinyl boronate complexes, says the new reactivity is intriguing. “It will be exciting to see what other types of strained, or even unstrained, ring systems engage in this type of reaction,” he says.
Aggarwal says they’re eager to explore other classes of bicyclobutane compounds in a range of reactions.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter