Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Zeolite intermediates offer new possibilities in catalysis
Amidst a well-known zeolite phase transformation, researchers have found active species that accelerate acid-catalyzed reactions
by Fernando Gomollón-Bel, special to C&EN
March 21, 2022

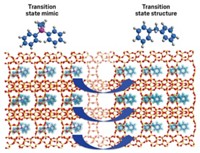
To make stable zeolites—porous inorganic materials used as industrial catalysts—researchers often convert the zeolites’ internal structure, transforming looser phases into more stable, denser forms. Now, chemists have caught these zeolites mid-transformation and discovered that the resulting intermediates supercharge three reactions important in industry (J. Am. Chem. Soc. 2022, DOI: 10.1021/jacs.2c00665). The process is highly tunable, giving chemists more control over the properties of their catalysts.
“Interzeolite transformation is a well-known process in the field,” says Javier García Martínez, whose lab at the University of Alicante conducted the study with collaborators at the National University of Colombia. “We decided to stop the process at different times and test the catalytic activity of the intermediates,” he says.
García Martínez’s team used three established methods to obtain the interzeolite transformation intermediates (ITIs): using an organic template, a surfactant, or a combination of both strategies. All are standard procedures for manufacturing mesoporous zeolites on industrial scales. The combination method creates a competition between the organic template and the surfactant, transforming zeolites considerably more slowly, which could be an advantage for controlling and monitoring the formation of ITIs, García Martínez says.
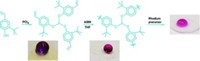
The researchers tried out the ITIs as catalysts in three popular acid-catalyzed reactions, including a Friedel-Crafts alkylation, a Claisen-Schmidt condensation, and polystyrene cracking. Overall, the turnover rate of the new catalysts was up to six times as fast as commercial zeolites.
Because the materials aren’t given time to fully set into the new structure, their pores are larger, thus allowing molecules to easily penetrate the porous network. Plus, the ITIs’ strongly acidic groups are more exposed, accelerating the acid catalysis, García Martínez says. “This is exactly why the ITIs showcase great activity.”
“Some people had tried to engineer similar catalytic materials, but this process looks more reproducible,” says Raúl Lobo, an expert in zeolites and materials engineering at the University of Delaware who was not involved in the study. Lobo points to the advantage of the improved control over the transformation and the uniformity of the new catalysts’ pores.
Matteo Cargnello, who studies materials for catalysis at Stanford University, says, “This synthesis could be easily adapted to large scales.” Companies could consider the added cost of the surfactant if their productivity increases, he adds.
In the meantime, García Martínez and collaborators have applied for a patent on the technology. “The possibilities of processing polystyrene into hydrocarbons have already attracted a few suitors,” he says.
CORRECTION:
This story's reaction scheme was updated on March 23, 2022, to show the correct tautomer of the reaction product. The product should have a hydrogen atom bonded to its nitrogen atom, not to the carbon atom in the indole ring's 3 position.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter