Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
How to make aziridines from olefins without expensive metals
Double bonds form nitrogen-containing 3-membered rings thanks to a reactive, yet fleeting, oxaziridine intermediate
by Bethany Halford
February 27, 2020

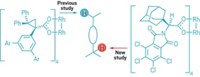
NH-Aziridines—those strained three-membered rings made up of two carbons and a nitrogen—are attractive targets for chemists. On their own, they have the potential to boost bioactivity in certain molecules. With the help of a nucleophile, they can spring open to form amines, which are often potent biomolecules. Describing work that should make these useful rings more accessible, chemists led by Rice University’s László Kürti now report a new method for making them. The synthesis is enantioselective, uses unactivated olefins, and doesn’t require expensive transition-metal catalysts (Nat. Catal. 2020, DOI: 10.1038/s41929-020-0430-4).
Kürti confesses to a longstanding fascination with NH-aziridines and their synthesis. Researchers in his lab previously made them using rhodium-based catalysts. But he points out that the price of rhodium has skyrocketed since that 2014 report, from less than $40 per gram to more than $400 per gram, putting the reagent out of reach for many researchers.
Kürti sought a less expensive method for making NH-aziridines, which meant ditching the metal and its mechanism. Inspired by a method for making epoxides—three-membered rings with two carbons and an oxygen—developed by Colorado State University’s Yian Shi, Kürti reasoned he could develop a similar route using nitrogen instead of oxygen. His group discovered that a transient, highly reactive NH-oxaziridine intermediate (made from an electron-deficient ketone and a nitrogen source) akin to Shi’s dioxirane can transfer nitrogen to unactivated olefins (example shown). The group collaborated with Peking University’s Xinhao Zhang to validate this mechanism using a modified mass spectrometer.
The new method has other advantages, too: If Kürti and coworkers use a chiral ketone to make the NH-oxaziridine, they can control the stereochemistry of the NH-aziridine, something that chiral rhodium catalysts can’t do. “With a single transformation I can turn olefin commodity chemicals into a much higher value material,” Kürti says.
“This constitutes a green, metal-free, and low-cost protocol that can be performed in any place around the world,” says Christoforos Kokotos, who studies metal-free catalysis at the University of Athens and was not involved in the research. “This is an outstanding contribution.”
The method has limitations, however. It doesn’t work on hindered olefins or some styrenes—olefins adjacent to an aromatic ring. It also doesn’t work on terminal olefins, but that’s something Kürti wants to tackle next.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter