Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Metal-free? The mistake that chemists seem doomed to repeat
A reaction touted to run without metals turned out to have trace amounts of palladium. It wasn’t the first time. How can research journals and chemists avoid making this mistake again and again?
by Leigh Krietsch Boerner
February 13, 2022
| A version of this story appeared in
Volume 100, Issue 6

Credit: Daria Kirpach
In brief
A high-impact journal published a paper claiming to have performed a long-sought-after metal-free coupling reaction. A wave of skeptical comments from Twitter provoked multiple follow-up papers that debunked the claims. The paper was ultimately deemed inaccurate, and the authors retracted it. This case is far from the first time that chemists published research making similar claims, which the community later found to be incorrect. But in most of the past cases, the researchers published follow-up papers, and the original research was not retracted, corrected, or marked as inaccurate. Chemists want to find ways to correct the scientific record and to avoid similar problems in the future.
The reaction to synthetic chemist Hua-Jian Xu’s article started like a dripping faucet.
Nature Catalysis published Xu’s paper online on Jan. 18, 2021 (DOI: 10.1038/s41929-020-00564-z), and over the next few days, there were a handful of mentions on Twitter. By Jan. 27, these drips had started to puddle, and then to pool.
Chemists did not believe the claims the authors were making.
In the paper, Xu and his colleagues reported a way to form new carbon-carbon bonds via the popular Suzuki reaction, also known as Suzuki-Miyaura coupling. Historically, this reaction has required a palladium catalyst; Xu’s group claimed to have done the chemistry without the metal. Organic chemists have long sought a metal-free Suzuki reaction because Pd is expensive. Also, discovering metal-free coupling reactions would be an intellectual milestone for synthetic chemists.
One of the early Twitter skeptics was Robin Bedford, a catalytic chemist at the University of Bristol. When a colleague sent him a copy of the paper, Bedford felt some déjà vu. “It was a sense of ‘Here we go again,’ ” Bedford says.
Bedford has worked with Pd chemistry for over 25 years, and he’s seen other groups make similar claims. In each of those instances, chemists eventually found that the reactions were not actually metal-free and instead were contaminated by small amounts of catalytic metals. Bedford and other chemists on Twitter wondered if the same thing was happening with Xu’s chemistry.
These chemists noticed that Xu and coworkers had used a Pd catalyst to make the amine that they then used to run the coupling reaction. They wondered if the metal could still be hanging around during the coupling reaction.
Three separate groups tested that hypothesis and published their results in follow-up papers on ChemRxiv: Gergely Tolnai and a group from Eötvös Loránd University on Feb. 23, Bedford and multiple coworkers on March 19, and Kazunori Koide and a small group from the University of Pittsburgh on April 16. All three groups could replicate Xu’s findings by using the paper’s reported methods. But the three groups also found that a Pd compound that formed during amine production somehow hung around, contaminating the coupling reaction. The groups each tried different ways to make the amine to eliminate this Pd contamination. The results were the same once they got rid of the contamination: subsequent Suzuki reactions did not work. The metal-free reaction wasn’t metal-free after all.
On Dec. 2, Nature Catalysis published all three ChemRxiv papers as peer-reviewed Matters Arising articles, Nature’s format for postpublication commentary on research papers. On Dec. 8, Nature Catalysis published the authors’ retraction notice, along with an editorial discussing the self-correcting nature of science. “This form of scrutiny is a precious resource for the community,” the editorial says, “and is essential to preserve the integrity and value of the scientific record.”
As chemists like Bedford commented on Twitter, Xu and his group aren’t the first chemists to deal with a catalytic metal contaminant affecting their chemistry. But in most of those other cases, the papers have not been retracted. When a paper is retracted, that notice becomes a permanent part of the scientific record. Without this step, there’s no prominent way for scientists to know that a paper contains science that doesn’t work. This lack of information leads some scientists to publish their own work on the basis of faulty chemistry.
These recurring cases of metal catalyst contamination point to a gap in how journals and scientists correct the scientific record. Chemists note that there are no widely adopted rules for when to retract a paper. Some want to find ways to make retractions more acceptable in the chemistry community, and therefore more common, so that researchers don’t continue to make the same mistakes. Without a way to note flawed papers in the scientific literature, some chemists worry that researchers will continue to make the same mistake over and over.


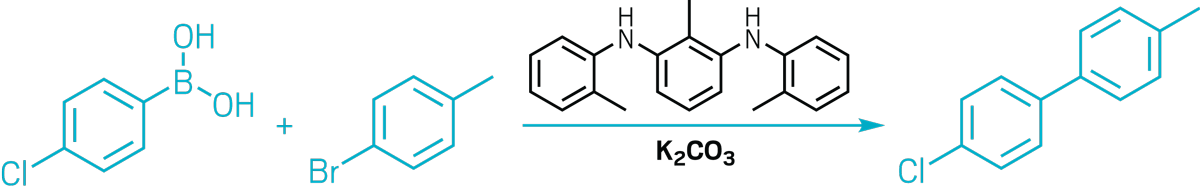
▸ Claim: Metal-free Suzuki reaction
▸ Impurity: Palladium in amine catalyst
▸ Follow-up action: Eleven months later, Nature Catalysis published three follow-up studies as Matters Arising. The authors retracted their paper 6 days after this.
▸ Notable paper: Xu, Lei, et al. “RETRACTED ARTICLE: The Amine-Catalysed Suzuki-Miyaura-Type Coupling of Aryl Halides and Arylboronic Acids.” Nat. Catal. (Jan. 2021). DOI: 10.1038/s41929-020-00564-z.
Not the first time
Probably the most well-known case of a supposedly metal-free reaction foiled by metal contamination is from the lab of Nicholas Leadbeater. In 2003, the organic chemist published two papers on a high-temperature microwave method for metal-free Suzuki coupling. Shortly after those papers came out, he and his group moved from King’s College London to the University of Connecticut. They then ran into a jarring problem: they couldn’t replicate the reaction.
“We tried everything,” Leadbeater says. “And it just wouldn’t work.”
They eventually discovered that in the UK, the sodium carbonate base they bought for their coupling reaction contained about 50 ppb of Pd. In the US, the base didn’t have that Pd contamination. This tiny amount of Pd was enough to catalyze their Suzuki coupling. Alarmed, the researchers revised their reaction method to instead use very low levels of a Pd catalyst. In 2005, they published the updated procedure in a separate paper.
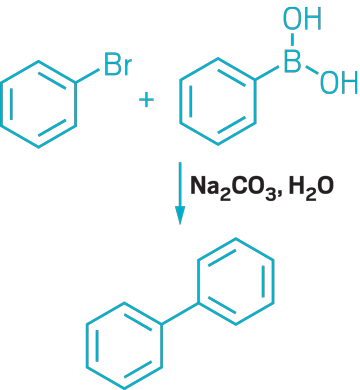

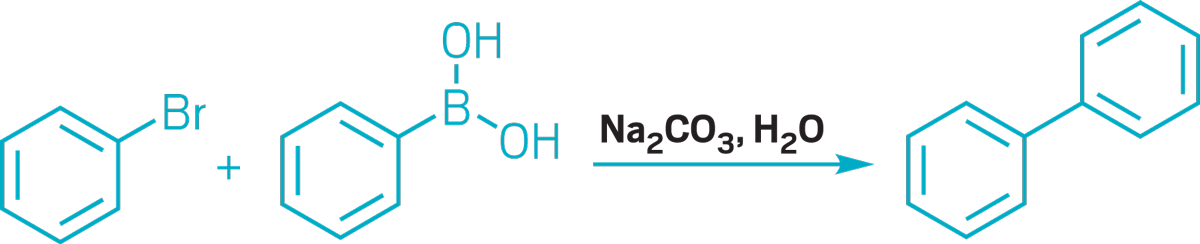
▸ Claim: Metal-free Suzuki reaction
▸ Impurity: ~50 ppb palladium in the Na2CO3 base
▸ Follow-up action: Two years later, the researchers published a revised method of the reaction using very low amounts of Pd catalysts.
▸ Notable paper: Leadbeater, Nicholas E., and Maria Marco. “Transition-Metal-Free Suzuki-Type Coupling Reactions.” Angew. Chem., Int. Ed. (March 2003). DOI: 10.1002/anie.200390362.
Leadbeater’s research was the first on record that claimed a metal-free Suzuki coupling. Other groups have made a metal-free claim or reported chemistry that used a different, less expensive metal as a catalyst. Most of these turned out to have metal contamination too. Leadbeater recently collected many of these examples in a book chapter. From his literature research plus his own experience, Leadbeater says, “If it seems too good to be true, it probably is too good to be true.”
Most of the contaminated research has involved either Suzuki coupling or Sonogashira coupling. The two reactions are similar: they both form new C-C bonds, essential to all aspects of organic chemistry, including drug synthesis and making green fuels. Both reactions also use a Pd catalyst. Suzuki coupling stitches together an organoborane compound with an organic halide or triflate compound, with help from a base. Sonogashira coupling also uses an organic halide, which binds to a terminal alkyne in a solution, and often also uses a copper iodide cocatalyst and an amine.
These coupling reactions are easy to run and often produce the desired product in good yields, so chemists tend to use them a lot, says Zoltán Novák, a catalytic chemist at Eötvös Loránd University who coauthored one of the Matters Arising papers with Tolnai. “They’re actually quite easy to do. You only need traces of palladium and this thing’ll go,” Leadbeater says. “You could do a Suzuki reaction in your bathtub at home if you wanted to.”
But because of the ever-growing cost of Pd, chemists have been trying for a long time to either switch to a different metal or ditch the metal entirely. In some parts of industry, chemists are running Suzuki reactions on 1,000 metric-ton scales, Tolnai says. “If you can switch Pd to something else that’s a little bit cheaper, just 0.01% cheaper, then you can save a lot of money,” he says.

But Pd is difficult to dump. Chemists struggle both to devise chemistry that can do what Pd does without the metal and to physically remove it from their reaction flasks. The metal sticks to everything: glassware, stir bars, spatulas, anything used in the reaction. As a result, Pd contamination in the lab can be a significant issue.
In Xu’s case, the contamination came from an unusually stable Pd complex left over from making the amine. The group used a typical Pd scavenger to pull any leftover metal from the amine, but the Pd complex was too stable, Xu says in an email. Instead, some Pd lingered in the amine reagent that the researchers then used to catalyze the Suzuki coupling. “We have spent nearly four years on this,” Xu says. And it was bad luck, Bedford says. Had the team used another ligand for the Pd catalyst or run a Pd-free control reaction, “none of this would have come to pass,” Bedford says.
The sources of metal contamination have varied in the previous cases. Some of these errors are due to carelessness, Leadbeater says. Others pop up because some chemists don’t do the necessary due diligence when it comes to analyzing their reactions for surreptitious metals. Often the researchers create a rationale for why their metal-free chemistry works instead of looking for lurking metal catalysts, he says. But later, they have to either retract the paper or do more follow-up research because it turns out to be wrong, Leadbeater says.
Correcting the record
When scientists find that one of their papers has errors, they generally have four options: retract it, correct it, ignore it, or publish a follow-up paper explaining what happened. Chemists have been more likely to retract a paper in recent years than 10 or 20 years ago. In many of the older metal contamination cases, researchers chose the follow-up paper approach. But when scientists write a follow-up paper rather than retract or correct their paper, it’s difficult for other chemists to know that there was a problem with the initial paper. It’s still out in the literature, with no indication that the chemistry is flawed.
When a paper is retracted, it means that the research should no longer be considered reliable by the scientific community, says Ivan Oransky, who cofounded the blog Retraction Watch. Retracted papers don’t get removed from the scientific record, but the journal marks it as retracted and typically posts a retraction notice that explains why the science in the original paper was flawed.


By contrast, follow-up papers don’t amend the original papers to indicate problems with the science. Scientists know about problems with the initial research only if they also read the follow-up paper.
This practice has created an uncomfortable dichotomy, Bedford says. “Why did some articles get retracted and some not?” What’s more, writing a follow-up article leaves a hole in the scientific record, which has consequences.
Advertisement
Bedford points out that both of Leadbeater’s initial papers claiming a metal-free Suzuki reaction are still in the scientific record, uncorrected. By early 2022, the papers had been cited a combined 364 times, some as recently as 2018. And while it’s difficult to know in what context every paper refers to the flawed paper, some definitely refer to the metal-free aspect. In these situations, flawed science begets more flawed science. Before Xu’s paper was retracted, materials chemists from Huazhong University of Science and Technology used Xu’s “metal-free” Suzuki method to make conjugated polymers. They published this research in October in Chemical Science. As doubt about Xu’s chemistry swirled online, the Huazhong authors retracted their paper on Nov. 30.
Even a correction can be problematic, Bedford says. “I look at the word correction to an article and I think they’ve missed a reference, or they might have got one of their figures wrong,” he says. Basically, he worries that people read such notices to mean that there’s a small mistake and not something larger, like the conclusions of the paper aren’t valid. If a paper’s underlying science is wrong, it should be retracted, he says. “But it isn’t always.”
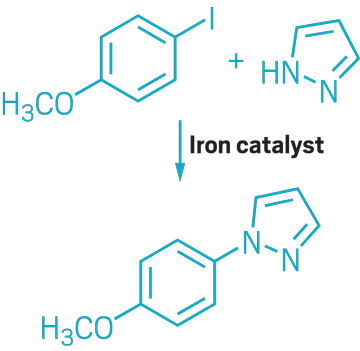

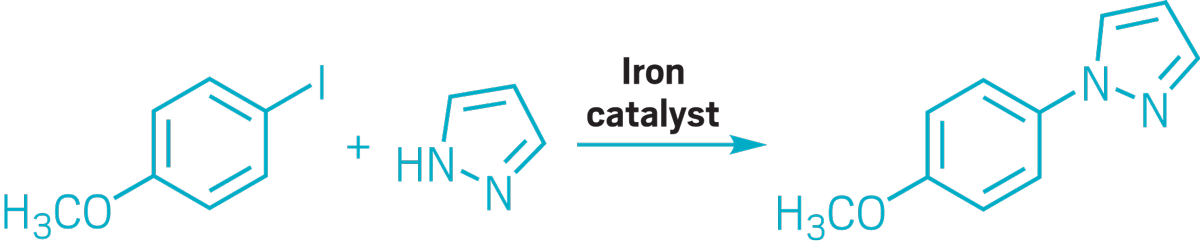
▸ Claim: Iron-catalyzed N-, S-, and O-arylation
▸ Impurity: Trace amounts of copper in FeCl3 catalyst
▸ Follow-up action: The next year, author Carsten Bolm published a joint paper with Stephen Buchwald showing that the reaction does not proceed without small amounts of Cu.
▸ Notable paper: Buchwald, Stephen L., and Carsten Bolm. “On the Role of Metal Contaminants in Catalyses with FeCl3.” Angew. Chem., Int. Ed. (July 2009). DOI: 10.1002/anie.200902237.
A new system?
Because there isn’t a standard way for journals to handle errors like those in these metal-free coupling papers, some chemists wonder what’s keeping this same mistake from happening again. Bedford is pretty sure it will. “I’m old enough and gnarly enough now to have seen it more than once.” He has a public bet that the chemistry community will see another case of erroneous metal-free chemistry after about 10 years.
So why aren’t more chemists retracting these older metal-free papers to prevent the mistakes from happening again? Technically, nothing is stopping authors from going back and requesting that older, incorrect papers be retracted now, Oransky says. Retractions to 20-year-old papers are not unheard of, he says. However, this idea may not catch on.
When asked about retracting his original metal-free paper, Leadbeater says via email, “We decided back then to publish our reassessment and my thoughts remain the same regarding that step.” He declined to comment on the matter further.
There’s no official party that oversees what gets retracted and what doesn’t, Oransky says. The Committee on Publication Ethics (COPE) “is the only game in town,” he says. That organization has a set of guidelines that advise journals to retract a scientific article when editors have clear evidence that the findings are not reliable, either due to a major error, or from fabrication or falsification. However, COPE can’t enforce these guidelines. “COPE doesn’t have any authority. It was never intended to have any authority,” Oransky says. “It’s a membership organization for journal editors and publishers. And so their goal is to create a set of best practices, not just for retractions, but for many aspects of publishing.”
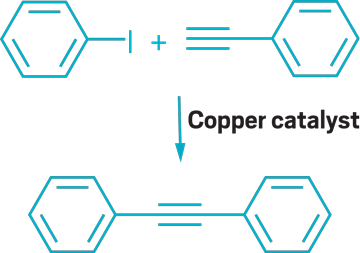


▸ Claim: Copper-catalyzed Sonogashira coupling
▸ Impurity: Trace amounts of palladium possibly from Cu catalyst, reagents, or glassware
▸ Follow-up action: A research group not affiliated with any of the original researchers published a follow-up paper.
▸ Notable paper: Gonda, Zsombor, Gergely L. Tolnai, and Zoltán Novák. “Dramatic Impact of ppb Levels of Palladium on the ‘Copper-Catalyzed’ Sonogashira Coupling.” Chem—Eur. J. (Sept. 2010). DOI: 10.1002/chem.201001880.
Over the past several years, COPE has started to threaten to kick publishers out of the organization if they don’t follow their guidelines, but that practice is still evolving, Oransky says. But in general, scientific publishing is a completely unregulated industry, he says. “It’s why people can continue just publishing nonsense. It’s why people can cite retracted papers” or not retract them in the first place. “There are just no sanctions for any of that,” Oransky says.
The correcting-the-record problem isn’t just on journals. There’s a stigma to getting a paper retracted that makes many people hesitate to choose that option. People tend to equate retraction with fraud, Oransky says. “About two-thirds of the time, misconduct is the reason for retraction,” he says. “So either you made it up, you made it look better than it really is, or you plagiarized.” For most of the remaining one-third of retracted papers, the cause is an honest mistake, such as authors realizing after publication that they used the wrong reagent. “Frankly, those are ones we should cherish, and those are ones we should talk more about because they’re the way things are supposed to work,” Oransky says. But the cases of misconduct tend to get more attention, he says.
Xu’s paper “is a classic example of a mistake happening. It’s done with good faith,” Bedford says. In cases like this, he believes that authors shouldn’t face negative consequences, such as loss of funding or people not trusting their science. “In an ideal world, a retraction of a paper like this would have no repercussions on the authors and should instead serve as a really good instance of how science should work,” Bedford says.
He suggests that there should be a different term for a paper retracted because of fraud to distinguish it from a retraction due to a mistake. Then the term retraction “wouldn’t come with the stigma of lumping you in with people who’ve made stuff up,” Bedford says. Finding mistakes is part of the scientific process, he says, and it needs to continue to happen.
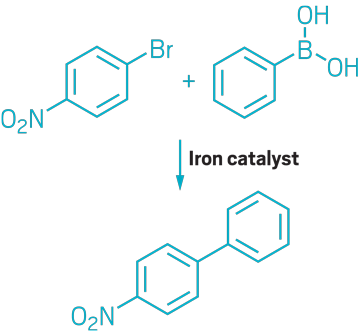

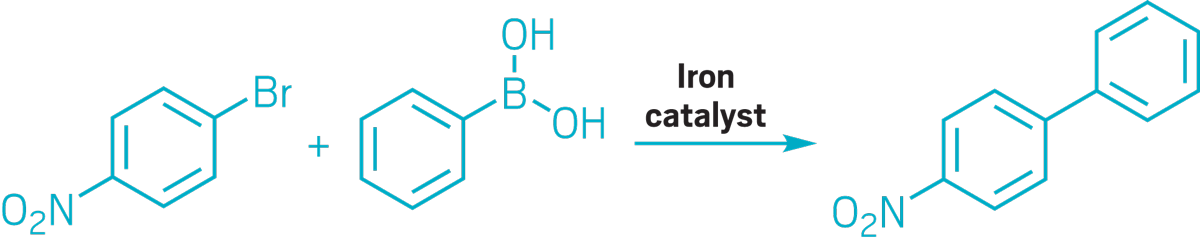
▸ Claim: Iron-catalyzed Suzuki coupling
▸ Impurity: Trace amounts of palladium in potassium fluoride reagent
▸ Follow-up action: A year later, the authors retracted the paper, and researchers not affiliated with the original research group published a follow-up paper.
▸ Notable paper: Kylmälä, Tuula, et al. “RETRACTED: trans-Tetrakis(pyridine)dichloroiron(II) as Catalyst for Suzuki Cross-Coupling in Ethanol and Water.” Tetrahedron Lett. (Sept. 2008). DOI: 10.1016/j.tetlet.2008.09.059.
Avoiding the mistake
Besides correcting the record for these cases of metal contamination, researchers who’ve seen this happen multiple times say that there are things chemists can do in the lab to avoid making similar errors.
“Most people probably don’t realize how active Pd is,” Bedford says. Because many people use more than they need for a catalytic reaction, their idea of the amount of Pd needed to catalyze a reaction is skewed. “Most of the time you get away with ppm levels, very happily, in those reactions. And because that’s not widely understood, people don’t know the extreme measures you have to go to to prevent Pd contamination,” he says.
The University of Pittsburgh’s Koide points out that it can be hard to prepare totally Pd-free samples in labs where the glassware has been repeatedly exposed to the metal. Leadbeater suggests chemists go to extremes when trying to avoid metals in the lab. That means separate fume hoods and balances for Pd chemistry and using new glassware and stir bars for every reaction. This can get expensive, Bedford says, but it helps avoid being tricked by lurking metals.
Bedford suggests chemists keep the metal away from every part of the reaction, not just the catalysis step. Koide also suggests systematically adding bits of the metal to “see if there’s a correlation between the different concentrations of Pd and the reaction rate,” he says.
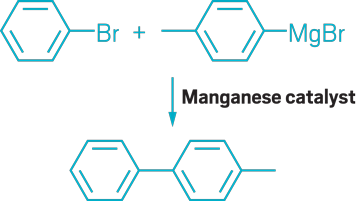

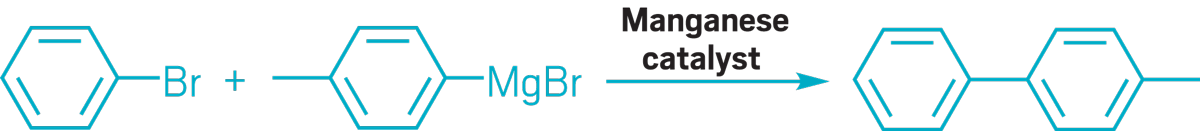
▸ Claim: Manganese catalysis of Grignard reagents with aryl halides
▸ Impurity: Trace amounts of palladium or copper
▸ Follow-up action: One month later, the researchers published a follow-up paper saying that they could not reproduce the earlier Mn catalysis research of either their or other groups.
▸ Notable paper: Antonacci, Giuseppe, et al. “Manganese-Catalyzed Cross-Coupling of Aryl Halides and Grignard Reagents by a Radical Mechanism.” Eur. J. Org. Chem. (July 2017). DOI: 10.1002/ejoc.201700981.
In doing metal-free or alternative-metal reactions, chemists need to go all out to prove themselves wrong, Eötvös Loránd University’s Tolnai says. Doing that can be hard, he says, because “how far do you want to go to prove yourself wrong?” Getting external feedback is also very valuable, Novák says. He points out that it was the feedback of other chemists that discovered the error of Xu’s group.
Despite all the bumps in the road to metal-free Suzuki and Sonogashira reactions, Bedford still hopes to see one or both legitimately happen one day. Tolnai points out that it is positive, not negative, that chemists make mistakes along the way to this goal, because the chemistry community can learn from these mistakes and move forward.
Xu says the experience with his paper and subsequent retraction shined a spotlight on how difficult it can be to try to replace Pd with organic catalysts. He’s not giving up on the chemistry. “We will also continue to explore this difficult research field and strive to make our contribution,” Xu says.
Chemists really would love to do these coupling reactions without expensive metals. “Maybe someone will make it,” Tolnai says. “We would never say not to try it.”






Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter