Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
How to optimize precious metal usage in catalytic converters
A study that tracks structural changes that deactivate and regenerate catalysts suggests ways to improve these important auto parts
by Mitch Jacoby
March 19, 2022
| A version of this story appeared in
Volume 100, Issue 10

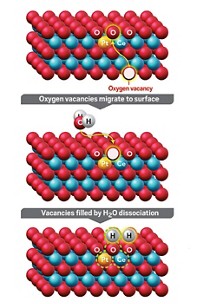
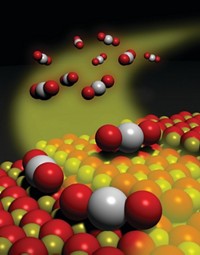
Catalytic converters efficiently strip pollutants and smog-forming compounds from engine exhaust by reacting these species on the surface of precious metals such as rhodium. The expensive metal may last longer and catalyst makers may be able to use it more sparingly thanks to a study that details atomic-level changes that deactivate and reactivate the catalytic metal (Chem. Mater. 2022, DOI: 10.1021/acs.chemmater.1c03513). Gasoline engine emissions are scrubbed by a three-way catalyst (TWC), which takes its name from the system’s ability to scrub three pollutants. TWCs oxidize hydrocarbons and carbon monoxide and reduce nitrogen oxides. Modern TWCs rely on rhodium nanoparticles typically supported on alumina. Earlier studies showed that exposure to oxidizing exhaust gases at high temperature can deactivate the catalyst and that reducing conditions can help restore its activity. But details of these processes have remained elusive. So Cheng-Han Li and Joerg R. Jinschek of the Ohio State University and coworkers at Ford Motor scrutinized TWCs using atomic-resolution microscopy, X-ray spectroscopy, and other methods. They exposed the catalysts to high temperatures and typical exhaust streams, which vary from oxygen rich to oxygen poor during normal engine operation. They showed that oxidizing conditions can cause rhodium nanoparticles to dissolve into the support and form rhodium aluminate, a catalytically inactive material. Reducing conditions help reverse the process. The study suggests that rhodium usage can be optimized by chemically anchoring the nanoparticles more tightly and by using alloys that resist dissolution.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter