Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Medicinal Chemistry
2 new syntheses lead to elusive pyridine compounds
Chemists functionalize tricky meta position by breaking pyridine’s aromatic structure
by Leigh Krietsch Boerner
November 17, 2022
| A version of this story appeared in
Volume 100, Issue 41
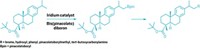
Pyridines, molecules consisting of one nitrogen and five carbons in a six-membered aromatic ring, are an important chemical stepping stone for a variety of drugs, agricultural products, and materials. These molecules are hard to modify, however, particularly at the meta position—or two spots over from the N—in the ring. This forces chemists to use high heat, strong acids, or metal catalysts to coerce pyridines to react. Now, two groups of scientists have developed different techniques to take pyridines apart and put them back together with new functionality at the meta position without using harsh reaction conditions. Both are mild, one-pot methods that may offer an easier way to create important commercial compounds and materials.

In the two approaches, the overall simplified reaction looks the same: start with a pyridine compound and end with a meta-substituted pyridine (shown). Chemists have a notoriously hard time adding functional groups to pyridines because the ring is electron deficient, says Andrew McNally, an organic chemist at Colorado State University and member of one of the research teams. Chemists can generally use electron-starved groups called electrophiles to add onto aromatic organic compounds. But because the N on pyridine pulls electron density away from its neighboring C atoms, electrophiles are reluctant to attack at these positions. The two teams used a similar approach of interrupting the aromatic system of the pyridine to get it to react at the desired position. “This is a general strategy, which allows us to broadly address the meta position using different kinds of reactions,” says Armido Studer, an organic chemist at University of Münster, who led the other team.
McNally and his team cracked open their pyridine compounds by modifying a technique published in 1904 called the Zincke reaction (Science 2022, DOI: 10.1126/science.add8980). The researchers used the electrophilic trifluoromethanesulfonic anhydride to break a C–N bond and create a linear compound. This effectively turns the pyridine from an electron-deficient system into something more electron rich, McNally says. The group then can add a halogen and close the ring back up. “It’s kind of like doing surgery on the molecule,” McNally says. “You rip this thing open, do your surgical intervention, and then you stitch it back together again.”
Instead of opening up the pyridine ring, Studer and coworkers converted their starting compounds to a stable oxazino intermediate and developed two paths to the functionalized pyridine (Science 2022, DOI: 10.1126/science.ade6029). One path goes through a radical reaction to form trifluoromethylated and perfluoroalkylated compounds, whereas the second uses an ionic mechanism to install halogen groups. The researchers functionalized quinolines and isoquinolines in addition to pyridines.
Both teams showed that their respective methods work on several pharmacologically active components and at a late stage in the synthesis process. This is important when making drugs, because many chemists already have protocols for synthesizing compounds of interest, Studer says. Changing a functional group in an existing compound is easier and faster than creating a new synthesis that puts in a functional group at the beginning.
“Both contributions are remarkable in the sense that the authors managed to functionalize pyridine derivatives with exquisite levels of meta-selectivity by implementing smart, one-pot, multistep organic sequences, whilst avoiding the use of costly transition-metal catalysts,” says Rafael Gramage-Doria, an organic chemist at the Rennes Institute of Chemical Sciences. The two reactions could become platforms for future development of other functionalizations, says Xiao-Chen Wang, an organic chemist at Nankai University.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter