Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Process Chemistry
Subtle base swap yields big difference in Suzuki coupling
Subbing an acetate for a carbonate means a cleaner, simpler reaction
by Leigh Krietsch Boerner
December 23, 2022
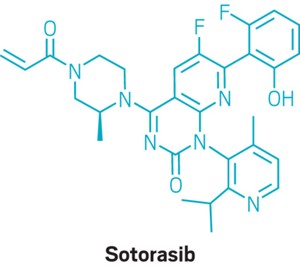
By looking very carefully at reaction rates and mechanisms, researchers have found a way to run a widely used reaction using less catalyst that results in better yields. A team at Amgen led by process chemists James Murray and Liang Zhang found that switching the base in the Suzuki-Miyaura reaction could also drastically reduce the amount of side product and make the reaction more controllable on a large scale (Org. Process Res. Dev. 2022, DOI: 10.1021/acs.oprd.2c00332). This finding is crucial to make many drugs safely and on a large enough scale to avoid shortages.
The Suzuki-Miyaura reaction, which uses a base and a palladium catalyst to make new carbon-carbon bonds, is one of the most widely used coupling reactions in industrial chemistry. By simply swapping the base from potassium acetate (KOAc) to potassium carbonate (K2CO3), the team eliminated the need to add one of the reactants slowly. Instead, they can add both reactants in the coupling reaction in bulk at the beginning. This allows for a simpler, more reliable reaction.
The team examined the overall process to make sotorasib, also known under the trade name Lumakras. This compound is one of the few that can treat non-small cell lung cancers caused by a mutation in the KRAS gene, which was formerly thought to be “undruggable.” The Suzuki-Miyaura reaction is one of the key transformations of the synthesis of sotorasib, Zhang says. Through kinetic and mechanistic studies, the researchers found that the rate-determining step changes depending on which base they choose. When the team uses KOAc, the crucial step is a reductive elimination. Exchanging the base for K2CO3 shifts the important step to a transmetalation reaction, which allows the team to use less Pd catalyst and add both coupling reagents all at once, instead of adding one slowly over 90 min. Zhang says it was surprising that this subtle difference in bases could lead to such big differences in both the performance and mechanism of the reaction.
“People are using these pharmaceuticals to improve their lives and prevent disease,” Murray says. “We have to make sure that we’re manufacturing this [compound] in exactly the same quality every time.” That’s a real challenge, often underestimated by chemists outside process chemistry, he says. “Gaining insight into reaction mechanisms really allows us to develop the most robust and reproducible processes.”
“I think the lesson learned here is that more detailed knowledge early in the development [of pharmaceuticals] can never be overlooked,” says Jason Hein, an organic chemist at the University of British Columbia. “It’s a wonderful example of how stopping and digging deeply into the mechanism pointed the team at a curious process.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter