Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Reaction Mechanisms
Tying up topological chirality
Chemists develop new method to synthesize and separate chiral catenanes
by Laura Howes
April 11, 2019
| A version of this story appeared in
Volume 97, Issue 15

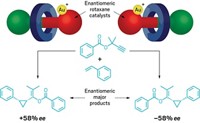
Linked molecules have long captured chemists’ imaginations, and their synthesis even won researchers the 2016 Nobel Prize in Chemistry. But while these molecules are often discussed in terms of molecular motors and switches, Steve Goldup’s lab at the University of Southampton is more interested in understanding how to make them. Now, his team has developed a method for synthesizing and separating topologically chiral catenanes, enabling investigations of the exotic molecules’ properties (Chem 2019, DOI: 10.1016/j.chempr.2019.03.008).
“The work is a major step forward,” says Nicholas Evans of Lancaster University. He says it’s the first demonstration of enantiopure samples of topologically chiral catenanes on a preparative scale.
Topological chirality refers to a higher-level spatial orientation in these molecules. On their own, the catenane loops are not chiral. When they are interlinked, their chirality depends on their orientations relative to each other. The only way the chirality could be flipped would be to open a ring and reform it in the other direction.
The synthetic trick is to create a precursor with a chiral auxiliary on one end before linking it round another loop in a copper-catalyzed reaction. Researchers use the chiral auxiliary to direct the stereochemical outcome and separate the two resulting molecules with flash chromatography. Finally, they remove the auxiliaries to produce pure enantiomers.
Interlocked compounds with chiral substituents have been used for asymmetric catalysis. Goldup hopes to find similar uses for topologically chiral molecules. “We don’t know what the benefits of topological chirality might be in catalysis because no one’s ever been able to study it,” Goldup says. “This is a newly available stereogenic unit. Let’s find out what we can do with it.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter