Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Reagents
Pirouetting reactor can run and purify multistep reactions
Simple system uses centripetal force to organize solutions of different densities for individual reactions
by Leigh Krietsch Boerner
September 30, 2020
| A version of this story appeared in
Volume 98, Issue 38

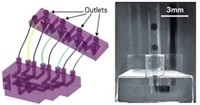
Chemists can automate sequences of chemical reactions using flow reactors, but these systems often need sophisticated engineering to set up and control. Bartosz Grzybowski and coworkers from Ulsan National Institute of Science and Technology and the Polish Academy of Sciences have designed a system that can run multistep reactions and separate out products without complicated fluidics and mixers (Nature 2020, DOI: 10.1038/s41586-020-2768-9).
In the new system, the team injects multiple solvents and solutions of different densities, both aqueous and organic, into an empty circular container. Spinning the container at a constant speed between 750 and 5,400 rpm forces the solvents to organize into discrete layers. When the researchers add a solution, it spins out to the perimeter. “Then you add another liquid, boom, it goes out and forms another layer inwards,” Grzybowski says. Keep adding layers and the liquids stack up like a wooden Russian doll, he says. The most dense solution forms the outer most concentric circle, while the least dense forms the inner most. The layers do not mix, even when they are as thin as 150 μm.
After the concentric circles have formed, the team can start injecting reagents into the layers to start the reactions. By designing the reactants and products to be soluble in different layers, the compounds migrate through the layers as each reaction occurs. For example, an organic compound added to a nonpolar solvent can react to form a salt, which would then move into a neighboring aqueous layer, where it could undergo another reaction to form a product soluble in the next-door polar solvent. “They’re going to react sequentially—A, B, C—going through different zones,” Grzybowski says. Essentially, the researchers can add starting materials to the center and collect their separated product from the outermost layer.
Using this system, Grzybowski and coworkers performed multiple reactions and separations, including the multistep syntheses of three small molecules: 1,4-phenylenediacrylic acid dimethyl ester, phenacetin, and diloxanide furoate.

In general, thinner layers lead to faster migration of the compounds. But, to speed up the reactions, Grzybowski and coworkers make quick changes to the container’s rotating speed. These speed changes make the individual layers swirl into each other temporarily. “There’s local mixing near the boundaries of the layers, which speeds up the transport from layer to layer without mixing the layers,” he says.
Centripetal forces form and maintain the organization of the layers, which is stable, “like a rock,” Grzybowski says, so unwanted mixing is unlikely. Unlike batch and flow systems, this new approach doesn’t need pipes or mixing devices. Researchers can easily control how much the layers will mix simply by varying the rotational speed.
The system can also shuttle compounds back and forth between layers, do simultaneous acid and base extractions between layers, and perform technically difficult separations of small molecules or nanoparticles.
The new method is a nice innovation with a lot of potential for small-scale, multi-step synthesis and diagnostics, says Lee Cronin, who studies complex chemical systems at the University of Glasgow. However, he points out that the system presents some challenges, such as getting the viscosity and density right for the various carrier solvents to effectively create discrete layers so reactions can occur smoothly.
The spinning reactor can potentially run more complex reactions, Grzybowski says. However, he says, reactions with a large number of steps might be tricky to design because chemists would have to find a large number of solvent systems with different densities and abilities to dissolve various types of molecules.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter