Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Microscopy
Pinpointing active sites for water splitting
Scanning probe method identifies defects in graphene-iron films that drive hydrogen evolution
by Mitch Jacoby
October 23, 2021
| A version of this story appeared in
Volume 99, Issue 39

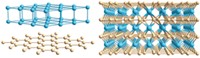
Microscopic defects on a catalyst made of carbon and iron actively drive the hydrogen evolution reaction, one of the key steps in liberating hydrogen from water, according to a study that combines electrochemical analysis with atomic resolution microscopy (Nat. Catal. 2021, DOI: 10.1038/s41929-021-00682-2). If scientists can find catalysts that efficiently split water into hydrogen and oxygen, then the oceans could serve as a nearly limitless supply of clean-burning, carbon-free hydrogen fuel for transportation and other uses. Precious metals work well as catalysts, but they’re expensive. So a team led by Stefano Agnoli and Gaetano Granozzi of the University of Padua examined inexpensive model catalysts consisting of thin films of graphene and iron. Sandwiches made from those films can be highly active electrocatalysts for hydrogen evolution, but how they work is unclear. The researchers used electrochemical scanning tunneling microscopy to examine the films while they mediated the catalytic reaction. By homing in on variations in the scanning tunneling current that track the catalytic process, the team probed individual sites on the films and identified the most catalytically active ones. Guided by quantum calculations, the researchers found that the most active sites include defects consisting of a few missing carbon atoms that exposed an underlying iron atom, as well as bent edges of graphene resembling carpeted steps. Such defects are easily generated by roughening the films.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter