Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Glutamate Transporter
Crystal structure hints at how brain controls levels of neurotransmitter
by Amanda Yarnell
October 18, 2004
| A version of this story appeared in
Volume 82, Issue 42

In the brain, neurons communicate with their neighbors by sending out bursts of small molecules known as neurotransmitters. Researchers have now cracked the structure of a microbial relative of the transporter protein that controls levels of glutamate, the most abundant neurotransmitter.
Neurons release glutamate in response to electrical impulses. Neighboring neurons detect the surge in glutamate and convert the message back into an electrical signal. For such signaling to continue, the glutamate must be cleared soon after it's released into the extracellular space. That job falls to a family of membrane proteins known as glutamate transporters.
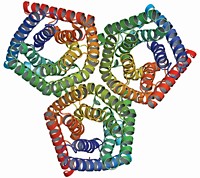
Because of the technical difficulties of crystallizing a human glutamate transporter, structural biologists Eric Gouaux, Dinesh Yernool, Olga Boudker, and coworkers at Columbia University turned to a heat-stable microbial relative identified by its similar sequence. The team's 3.5-Å structure--the first of any neurotransmitter transporter--hints at how the ones in humans work [Nature, 43, 811 (2004)].
The trimeric transporter is bowl shaped, with a water-filled extracellular basin that dips nearly halfway across the membrane bilayer. At the bottom of the basin, three pairs of helical hairpins create three glutamate-binding sites. Gouaux suggests that these hairpins act as gates that allow alternating access to either the extracellular or intracellular side of the membrane. Such a mechanism is necessary to prevent glutamate, which is present at much higher levels inside neurons than outside, from simply rushing down its concentration gradient and flooding the extracellular space. Gouaux hopes that structures of the transporter in other functional states will reveal the conformational changes required for glutamate transport.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter