Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
At last, terminal cyaphide ligand
September 18, 2006
| A version of this story appeared in
Volume 84, Issue 38
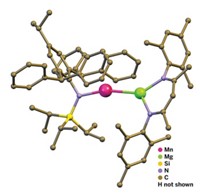
The quest is over for making an isolable cyaphide metal complex--a compound with a terminal -C≡P ligand, the phosphorus equivalent of cyanide. Following attempts by a number of chemists over several decades, Hansjörg Grützmacher and his coworkers at the Swiss Federal Institute of Technology, Zurich, finally devised a synthetic strategy capable of transferring the -C≡P moiety to a transition-metal ion (Angew. Chem. Int. Ed. 2006, 45, 6159). Phosphaalkynes (R-C≡P) and bimetallic complexes with a bridging ligand (M-C≡P-M) were previously known. The researchers first synthesized a silyl-substituted phosphaalkyne, Ph3Si-C≡P (Ph = phenyl), in which the silyl group functions as a protecting group. This phosphaalkyne was coordinated to a ruthenium bis(1,2-diphenylphosphinoethane) complex via the phosphorus atom. Reacting that complex with sodium phenoxide cleaved the silyl group and flipped the C≡P group to coordinate to ruthenium via the carbon atom, generating the cyaphide complex shown. The researchers hope their synthesis of Ph3Si-C≡P will be generally useful to make other cyaphide complexes, they note.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter