Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Largest Synthetic Hetero-Oligosaccharide
Milestone could lead to better understanding of major bacterial diseases
by Stu Borman
November 8, 2006

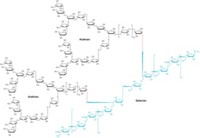
Researchers have succeeded in constructing the largest ever synthetic hetero-oligosaccharide, a carbohydrate containing different kinds of sugar units. The 28-unit structure, a lipoarabinomannan (LAM), is part of the cell-surface oligosaccharide of mycobacterial species that cause a range of diseases, including tuberculosis and leprosy.
The natural product cannot easily be derived in pure form from bacteria, so a synthetic route is desirable. The synthesized structure lacks sugar residues and linkages and a series of dangling lipids found in the complete natural product.
The synthesis could lead to a deeper understanding of the mechanism of mycobacterial infections and of LAM's biological activities, which include immunomodulatory effects and intracellular signaling. The work also could help lead to the synthesis of the entire lipidated cell-surface oligosaccharide, which contains 60–70 sugar residues.
The research was carried out by carbohydrate chemist Bert Fraser-Reid, postdocs Jun Lu and K. N. Jayaprakash, and visiting professor J. Cristóbal López at the Natural Products & Glycotechnology Research Institute, Pittsboro, N.C. (Tetrahedron: Asymmetry 2006, 17, 2449). The institute is a private, nonprofit research organization with laboratories at the Centennial Campus of North Carolina State University, Raleigh, N.C.
Fraser-Reid and coworkers earlier reported having synthesized the 12-sugar lipomannan segment of the cell-surface oligosaccharide (Angew. Chem. Int. Ed. 2005, 44, 5894; C&EN, Sept. 19, 2005, page 38), but this represented less than half of the new structure.
The rest of the new structure consists of a large arabinan domain, which includes a series of furanose sugars and a set of sugar "caps." The furanoses were the most challenging part of the synthesis. "Furanose sugars have received comparatively little attention," Fraser-Reid says. "Our methodology has revolutionized how these are put together."
The lipidless 28-mer structure the researchers have synthesized varies only a little among different types of mycobacteria, but the caps differ from one species to another and determine the oligosaccharide's biological specificity. In the synthetic LAM, Fraser-Reid and coworkers used mannose caps, the type present in Mycobacterium tuberculosis.
"Apart from this being a world record for hetero-oligosaccharide synthesis, it is also a superb piece of work," comments organic chemistry professor Steven V. Ley of Cambridge University, a specialist in natural product synthesis. "It's beautifully planned, and it really shows the power of modern-day oligosaccharide synthesis."
Synthetic carbohydrate chemist Todd L. Lowary of the University of Alberta says, "This is an impressive synthetic achievement. Access to oligosaccharide fragments of LAM using approaches such as this will be essential in providing a molecular-level understanding of the role of this polysaccharide in mycobacterial pathogenesis."
Professor of microbiology, immunology, and pathology Delphi Chatterjee of Colorado State University, who specializes in mycobacterial carbohydrates, notes that Fraser-Reid and coworkers incorporated α-linked furanoses in their structure instead of much more synthetically challenging β-linked furanoses present in the natural product. "So the synthesized molecule is not the real LAM structure present in the M. tuberculosis cell wall," she says. In the future, the researchers "will need to modify this molecular structure to match the real molecule in mycobacteria."
That said, the new synthesis is still "one of the most difficult and meaningful tasks in the history of carbohydrate chemical synthesis," Chatterjee adds.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter