Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Multitasking Catalysts
Mix-and-match enzymes can catalyze all four isoprenoid coupling reactions
by Celia Henry Arnaud
April 9, 2007
| A version of this story appeared in
Volume 85, Issue 15
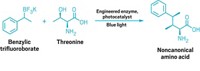
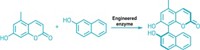
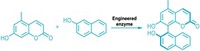
Enzymes made of bits and pieces of two other enzymes can catalyze all four reactions used to build the skeletons of isoprenoid natural products, chemists have discovered. The multitasking catalysts may yield clues about how nature evolved the ability to make these compounds.
Isoprenoids are the most chemically diverse family of natural products. More than 55,000 naturally occurring isoprenoids or terpenoids are known, with examples from all over the world. This broad family includes compounds that play important roles in metabolism and cell structure. Remarkably, this vast array of compounds is generated from simple precursors by a set of just four coupling reactions: chain elongation, cyclopropanation, branching, and cyclobutanation.
In nature, each reaction is usually carried out by a separate enzyme. In the course of studying these enzymes, chemistry professor C. Dale Poulter and coworkers at the University of Utah have created chimeras that can perform all four reactions (Science 2007, 316, 73). Starting with the chain elongation and cyclopropanation enzymes from a sagebrush plant, they replaced segments of the active site of the chain elongation enzyme with corresponding bits from the cyclopropanation enzyme.
"We're not changing the size of the protein, and we're not introducing extra active sites," Poulter says. "All we're doing is changing the amino acids used to construct the active site."
The unexpected discovery that some of the chimeras can perform all four of the coupling reactions could provide insight into how the natural enzymes that catalyze these reactions could have evolved from a single precursor. "Once you have the ability to do all four reactions, then making one pathway selective over another is basically what the job of evolution is about," Poulter says. The efficiency of the multitasking enzymes for the various reactions was dramatically different, he notes.
In an accompanying commentary, David W. Christianson, a chemistry professor at the University of Pennsylvania, writes that the "chimeras exhibit remarkable trends in biosynthetic versatility." In addition, the work provides "compelling evidence" that the enzymes "that catalyze these fundamental coupling reactions diverged from a common ancestor early in the evolution of terpenoid biosynthesis," Christianson writes.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter