Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
FDA aims at nanotech safety
July 30, 2007
| A version of this story appeared in
Volume 85, Issue 31
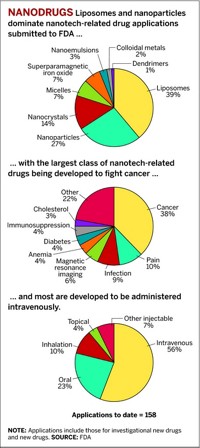
FDA's Nanotechnology Task Force on July 25 released a report recommending that the agency address nanotechnology risks. Engineered nanoscale particles increasingly are being used in medical devices, drugs, and cosmetics and are being developed for use in food, animal feed, and food packaging. Most of the product types FDA regulates could eventually contain nanoscale particles, the report says. Nanotechnology presents special challenges, it notes, because properties relevant to product safety may change as particle size decreases and because FDA does not yet have all the tools it needs to assess the effects of nanoparticles on the human body. The report recommends that FDA develop nanotech "guidances" that spell out what data manufacturers should give FDA about their products and when the use of a nanoscale ingredient changes the regulatory status of the material. FDA officials say the first guidances will be completed in a few months.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter