Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
No Sugarcoating For Insulin Failures
Major drug companies have a medical flop with inhaled insulin, but one biopharmaceutical firm keeps trying
by Ann M. Thayer
May 12, 2008
| A version of this story appeared in
Volume 86, Issue 19
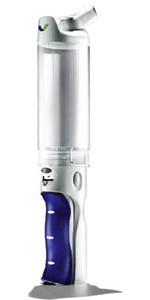
IT'S HARD TO FATHOM how a company that sells $60 billion worth of pharmaceuticals per year could misjudge a target market as badly as Pfizer did when it launched Exubera, its inhaled insulin product.
To be fair, Pfizer stands out largely for being the first to market inhaled insulin and the first to call it quits. Eli Lilly & Co. and Novo Nordisk spent a decade on R&D as well, but they pulled the plug on their products during late-stage clinical trials. Also involved were smaller technology partners that helped develop drug delivery devices. Only Valencia, Calif.-based MannKind retains any major commitment to inhaled insulin.
Intuitively, inhaled insulin makes sense. The market is enormous—an estimated 230 million people worldwide have diabetes, and those being treated used $24 billion worth of antidiabetic drugs in 2007, according to the market analysis firm IMS Health. More than a third of those products are various forms of insulin, which patients inject to control sugar levels in their blood. Anything that would avoid those injections sounds like an attractive alternative, so long as it is safe, effective, and convenient to use.
Pfizer and its partner, Nektar Therapeutics, took 10 years and created an effective and technologically advanced insulin delivery system that was considered safe for the majority of patients. Regulators welcomed it as the first new delivery option for insulin in 80 years. But patients found Exubera inconvenient and costly to use.
Exubera couldn't be used by children, smokers, recent ex-smokers, or anyone with a lung disorder. Side effects included small decreases in lung function that were reversible when the drug was stopped. But diabetes is a chronic disease, and some doctors had concerns about long-term use.
"Inhaled insulin was considered to have blockbuster potential but has turned out to be a flop," says Grant Zeng, an analyst with Zacks Equity Research in Chicago. In short, physicians weren't widely prescribing it, patients weren't receptive to it, and insurers weren't willing to cover all of the cost.
U.S. and European regulators approved Exubera in early 2006, but manufacturing problems delayed Pfizer's launch until several months later. Early on, Pfizer bullishly estimated that 2010 sales would hit at least $1.5 billion. In the first nine months of 2007, however, sales totaled just $12 million, and by October Pfizer had exited the market.
"After Pfizer decided to withdraw support for its product, Lilly and Novo didn't see any upside potential for theirs," Zeng adds. In January, Novo dropped its AERx insulin system, which was projected to gain approval in 2010. Then Lilly, which had expected its inhaled-insulin product to reach the market in 2009, cancelled its AIR insulin program in March. Like Pfizer, both companies emphasized that their moves were not related to any safety concerns.
On top of the more than $1 billion Pfizer had spent on R&D, the company took a $2.8 billion charge to write off the product. It had paid $1.3 billion in 2006 to buy out Sanofi-Aventis' share of what was then a three-way collaboration for Exubera. The deal included an insulin plant in Frankfurt, Germany. Total price tag: at least $5 billion
In an October 2007 conference call, Pfizer executives admitted that they underestimated physician and patient resistance toward switching to Exubera. One factor was what they called the "burden" the technology presented to the medical field.
That was their way of acknowledging that Exubera had problems. It was more expensive than injected insulin, in part because larger amounts of insulin were required to achieve the same therapeutic effect. The drug must be delivered deep into the lungs, and some is lost in the inhaler and in air passages. As a result, Exubera used about five times as much insulin as a typical injected dose and cost at least twice as much.
Moreover, before patients could use the tennis-ball-can-sized Exubera inhaler, they had to undergo lung function testing and training. Exubera also used powdered insulin doses measured in milligrams, rather than the more familiar international units. And many patients found the process of combining doses to be confusing because they couldn't add them together linearly.
YET PFIZER and its competitors had good reasons for trying to update insulin delivery. "If somebody comes up with a product that the doctors will prescribe, the patients will like, and the reimbursement authorities will reimburse, it will be used and it will be huge," says Igor Gonda, chief executive officer of Hayward, Calif.-based respiratory disease specialist Aradigm.
Aradigm had partnered with Novo to develop the AERx system. The companies started working together in 1998, but by the time Novo dropped the inhaled product, the relationship had evolved primarily into a licensing agreement. Novo made its last significant payment of development funding in 2005; Aradigm was to receive royalties on AERx insulin sales.
Novo had rights to use Aradigm's delivery technology in the diabetes field, while Aradigm could use it in other areas. Novo also had purchased manufacturing assets from Aradigm in 2005. Between 1998 and 2007, Novo paid Aradigm a total of about $150 million in product development and milestone payments and bought about $35 million worth of Aradigm stock.
But it wasn't just the clumsy delivery method that worked against Pfizer and the other developers of inhaled insulin. During the decade that these firms were in the lab, the market for diabetes therapies was changing dramatically.
In recent years, new options, including many orally administered drugs, have emerged for treating type 2 diabetes, the most common form of the disease, which arises when the body loses its ability to respond to insulin. These include Byetta from Amylin Pharmaceuticals, Januvia from Merck & Co., and Galvus from Novartis (C&EN, Jan. 8, 2007, page 30).
TYPE 1 DIABETES patients, who make up about 10% of the patient population, do not produce insulin and must inject it. Even for injectable insulin, producers have worked hard to improve delivery methods. Lilly, Novo, and Sanofi-Aventis all make insulin pens, small, easy-to-use devices that let patients discreetly carry and inject insulin. The pens have very short, fine needles that go far to help reduce the anxiety and pain associated with injections.
According to the American Diabetes Association (ADA), most patients overcome their resistance to injections. Diabetes patients typically use short-acting insulin at mealtimes and long-acting insulin to maintain baseline levels. Once accustomed to injections, patients may not see much benefit in an inhaled short-acting product such as Exubera if they still must inject long-acting insulin. But patients who are new to diabetes therapy might be attracted to inhaled forms if these met all their insulin needs. And once on inhaled therapies, these patients might be reluctant to switch to needles.
"We have concluded that fast-acting inhaled insulin in the form it is known today is unlikely to offer significant clinical or convenience benefits over injections of modern insulin with pen devices," Novo CEO Lars Rebien Sørensen said in announcing the exit in January. The company took a $375 million charge against earnings and laid off employees at its Novo Nordisk Delivery Technologies unit, the former Aradigm site. It also has dropped any plans to develop inhaled long-acting insulin and a glucagon-like peptide-1 (GLP-1) analog that promotes insulin production.

Gonda believes, however, that drug companies might be successful if they were to simultaneously develop inhaled forms of long- and short-acting injectable diabetes drugs. Inhaled insulin, he points out, is not meant for existing patients using injected insulin. "It is meant to be a replacement for the expensive oral hyperglycemic drugs, because for many patients they stop working," he says. "If you have a treatment that for safety or efficacy reasons or both is better than oral hyperglycemic drugs, then patients would surely choose it instead of injections."
Gonda adds that the Aradigm technology used by Novo differed from Exubera in a few key ways, not the least of which was its smaller size. It used an aqueous formulation that allowed the delivery of partial and more fine-tuned doses. "The device enables you to dial in how many doses of insulin you want to take in equivalent subcutaneous units," he says. The AERx device also electronically recorded usage and guided the patient through the proper breathing pattern.
Likewise, Lilly's AIR insulin device was designed to fit in the palm of the hand and deliver an effective dose of insulin safely and with limited side effects. Nevertheless, Lilly cited "increasing uncertainties in the regulatory environment" and "a thorough evaluation of the evolving commercial and clinical potential of the product compared to existing medical therapies" as reasons for its withdrawal. It took a $146 million charge against earnings, ended supply agreements, and cut back insulin production last month.
Lilly's partner, Alkermes, is not planning to pursue AIR insulin, a company spokeswoman says. Lilly will pay Alkermes an estimated $13 million to cover the "wind-down" costs.
Surprisingly, Lilly's decision to terminate the program came 14 months after the two companies had extended their collaboration to include the construction of a second production line to meet expected commercial demand. Since March, Alkermes has restructured its operations, closed its AIR insulin facility, and laid off 150 employees, or 18% of its workforce.
It's difficult to estimate how much Lilly has paid Alkermes regarding AIR because the companies also have other programs unrelated to insulin. Lilly's decision was a major blow to Alkermes, but the company has other products on the market and is already profitable, Zeng points out. Alkermes' drug delivery technologies for inhalation and controlled release are considered sound and are being applied to other products.
Likewise, Nektar, which was Pfizer's partner, survives with its pulmonary and PEGylation drug delivery technologies intact. Interestingly, the company generated more income from inhaled insulin than did Pfizer; for 2006 and 2007, Nektar's income related to Exubera R&D and manufacturing totaled $329 million, or two-thirds of its revenues. Pfizer also paid Nektar a $135 million settlement fee for Exubera and a next-generation product in early development.
Prior to the settlement, Nektar executives were displeased with their partner because they learned about the decision to drop the drug from Pfizer's press release. "Nektar has been very disappointed in Pfizer's performance in marketing Exubera," Nektar CEO Howard W. Robin said in an October 2007 statement.
As part of the deal, Pfizer agreed to transfer all rights if Nektar found a new partner. But those plans came to a screeching halt on April 9.
On that day, Pfizer reported that six out of 4,740 Exubera patients had developed lung cancer, compared with one in the 4,292-patient control group. Due to the limited number of cases, Pfizer says it can't be determined whether Exubera was to blame. All patients diagnosed with lung cancer had a history of cigarette smoking.
Nektar subsequently announced that it was ending all negotiations with potential partners. "Fortunately, over the past year Nektar has significantly transformed its business, moving away from inhaled insulin," Robin said in an April 9 press release. This shift included letting go about 20% of its employees and ceasing all insulin-related spending.
Because diabetes affects so many people, the regulatory bar has always been high. Inhaled insulin introduces additional concerns because as a growth factor it could play a role in cellular changes in the lung. And a study published recently by ADA points out that the lung is already a target organ for diabetic injury (Diabetes Care 2008, 31, 735). The use of inhaled insulin may "trigger or exacerbate pulmonary dysfunction," according to one of the study's authors.
Aradigm has since reported that no occurrences of primary lung cancer or abnormal cell proliferation were seen in Novo's AERx insulin studies.
ALTHOUGH the competition is gone, the situation doesn't bode well for MannKind, a biopharmaceutical company specializing in diabetes and cancer treatments. Over about 15 years, MannKind has invested almost $750 million in R&D largely for its Technosphere inhaled insulin, as well as on an early-stage inhaled GLP-1 and an early cancer drug. Much of the money has come from the company's CEO and founder, 82-year-old billionaire Alfred E. Mann.
Advertisement
MannKind President Hakan S. Edstrom believes it's a disservice to lump all inhaled insulins together. Safety problems with Exubera are specific to that product, he points out. "The other products have also shown that inhalation alone, and at a price premium, is not a strong enough argument to adopt this kind of therapy."
MannKind has developed a cell-phone-sized device that delivers consistent units of insulin. Moreover, Edstrom says, Technosphere has a very different pharmacological profile that even offers advantages over injected short-acting insulins. In particular, the Technosphere insulin acts very rapidly to synchronize with mealtimes and doesn't persist too long afterward, which would lead to hypoglycemia.
The Technosphere powder consists of a carrier to which the insulin is electrostatically bound at the monomeric level, allowing it to act faster, Edstrom explains. Made of fumaryl diketopiperazine, the approximately 2-µm carrier particles self-assemble with the insulin under slightly acidic conditions. When the particles hit an environment above pH 6.5, such as in the lung, they fall apart and the insulin is absorbed.
Edstrom says MannKind, which has a 2,000-patient pulmonary safety trial under way, has not seen a difference in lung function in its trials. "We are very comfortable at this point with regard to the safety profile," he adds. The company has completed toxicology and carcinogenicity testing. "We hope to start sharing data in late July or August," he says, regarding a 5,000-patient Phase III trial under way, with an ambitious target of filing for approval by the end of the year.
Nevertheless, recent events have hurt MannKind. In response to Pfizer's lung cancer news, MannKind suspended discussions with potential partners until its clinical data are available. "There is so much noise in the marketplace that may prevent us from getting the valuation we think this therapy offers," Edstrom says. Meanwhile, the company's conversations with FDA indicated it could proceed as planned, he adds.
Zacks's Zeng cautions that FDA may raise the bar for approval and ask for even more clinical data. MannKind is already spending tremendous amounts of cash and may have difficulty raising more if approval is delayed, he says. In early 2007, the company's stock price was close to $20 per share; it now trades at less than $3.00.
Diabetes is a complex disease that confounds drugmakers and the medical community by changing as it progresses. Yet companies continue to invest millions in trying to develop better ways to treat it. "There is still a huge unmet need and a rapidly growing market in treating diabetes," Edstrom says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter