Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Business
Capturing Carbon
Novomer aims to turn carbon dioxide into something useful
by Alexander H. Tullo
June 23, 2008
| A version of this story appeared in
Volume 86, Issue 25

IT SOUNDS TOO GOOD to be true. Ithaca, N.Y.-based start-up Novomer has a technology that reacts carbon dioxide with other molecules, locking it into a polymer backbone and putting it to better use than as an atmosphere-warming gas. The company says the anti-greenhouse-gas bonus of its chemistry has drawn attention, but it would rather focus its efforts on making polymers that people want to buy.
At the heart of Novomer's technology are catalysts developed by Geoffrey W. Coates, a chemistry professor at Cornell University. The catalysts are based on transition metals and can break open the highly stable CO2 molecule, enabling it to polymerize with epoxide groups. For example, CO2 combines with ethylene oxide to make the polymer polyethylene carbonate and with propylene oxide to make polypropylene carbonate. These polymers have about 50% and 43% CO2 content by weight, respectively.
Coates began the research that led to the catalysts when he arrived at Cornell in 1997, after previously studying olefin catalysis during his graduate studies at Stanford University and during his postdoctoral fellowship with Nobel Laureate Robert H. Grubbs at California Institute of Technology. He says the work of Donald J. Darensbourg at Texas A&M University inspired him. "One of his papers really excited me about using carbon dioxide as a building block," Coates says.
In 1998, Coates's group reported a catalyst, β-diiminate zinc acetate, that polymerizes CO2 and epoxides. In 2003, the group published a paper on salen cobalt carboxylate complexes, which Coates says increased the number of epoxides compatible with CO2. He and two colleagues founded Novomer in 2004.
Although the notion of fighting global warming has prompted many people to contact the company, Coates stresses that his technology is no panacea; humans generate way too much CO2. "If we took all the CO2 from burning coal and made plastic, we would make orders of magnitude more plastic than the world currently makes," he explains. Indeed, the Intergovernmental Panel on Climate Change says industrial use of CO2, including as a reactant to make urea, is about 115 million metric tons per year, only 0.5% of total anthropogenic emissions.
Novomer's president, Charles Hamilton, says the main benefit of using CO2 is its availability. "CO2 is a great raw material in that it is very cheap," he says. "In some cases you can get paid to use it."
John J. Murphy, vice president of the Spring House, Pa.-based consulting firm the Catalyst Group, stops just short of saying that polymerizing CO2 is the Holy Grail. "If you can unlock such an inert molecule and do something with it other than injecting it into aquifers, then you are onto something," Murphy says. But he notes a stumbling block for many a new material: "Does the market really need the product?"
This is why, instead of saving the world, Novomer is trying to match the properties of its polymers to market needs. "Ultimately, what determines the success of the materials is their performance and price," Hamilton says.
The company's first major commercial launch is a grade of polypropylene carbonate called NB-180, which stands for Novomer Binder 180 ºC, Hamilton says. At that relatively low temperature, the polymer chain breaks up and sublimates. Used as a binder, it can "burn off" without a trace when it is no longer needed. It is thus ideally suited for applications such as photolithography, the technology used to draw lines tens-of-nanometers wide on integrated circuits. Novomer is also looking at applications in capacitors, resistors, printing binders, and in binding diamonds onto saw blades.
Novomer could make coatings out of polypropylene carbonate, but Hamilton says the company isn't ready to tackle such large-volume applications just yet. "But NB-180 based on burn-off is a great product for us because it is an immediate market need," he says.
In the future, the company will tackle polyethylene carbonate, which Hamilton says has good oxygen-barrier properties and is suitable for making plastic films. He says the polymer may be an ideal gas-barrier laminate for use with polyethylene terephthalate in food and beverage packaging, such as soda bottles.
THE COMPANY is also exploring the properties of polycyclohexene carbonate, the result of polymerizing cyclohexene oxide and carbon dioxide. And it is looking into epoxides derived from biological sources, such as ethylene oxide made from ethanol.
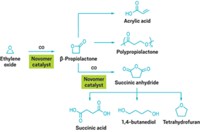
Novomer can use carbon monoxide as a building block as well. Hamilton says the carbonylation of carbon monoxide with epoxides yields lactones that can be used to make polyesters such as polyhydroxybutyrate.
the company has generated some commercial interest. Last October, Novomer raised $6.6 million in equity financing. DSM Venturing, the venture capital arm of the Dutch polymers and chemicals maker, participated in the round. "They are a great partner for us," Hamilton says. "They have the scale and the application development experience." Novomer is also collaborating with Eastman Kodak on new polymers.
Hamilton says Novomer is benefiting from fortuitous timing. "The board rooms of every major chemical company in the world are focused on what happens when petroleum runs out," he says. "Think of our chemistry as extending the chemistry of polymers beyond the petroleum era."



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter