Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Direct Allylic C–H Alkylation Solved
by Jessie Jiang, Contributing Editor
October 13, 2008
| A version of this story appeared in
Volume 86, Issue 41

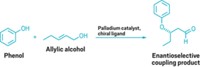
Direct allylic C–H alkylation—coupling a carbon of a nucleophilic molecule to an allyl group (a terminal alkene) of another molecule—is now possible without prefunctionalization by using a Pd(II)/bis(sulfoxide)/benzoquinone catalyst system, chemists from China and the U.S. report. Zhang-Jie Shi and coworkers of Peking University achieved intermolecular alkylations (shown) using allylarene substrates with dicarbonyl nucleophiles (J. Am. Chem. Soc. 2008, 130, 12901). They also showed that some allylic compounds can form cyclic products through intramolecular reactions. Separately, Andrew J. Young and M. Christina White of the University of Illinois, Urbana-Champaign, carried out intermolecular allylic alkylations using aromatic and heteroaromatic allyl substrates with carbonyl- and sulfonyl-based nucleophiles (J. Am. Chem. Soc., DOI: 10.1021/ja806867p). White's group discovered the Pd(II)/bis(sulfoxide)/benzoquinone catalyst system in 2004. Before these two reports, allylic alkylations were normally achieved in two steps through Pd(0)-catalyzed allylic substitution reactions that required installing a functional group at the allylic position. The direct conversion is expected to help streamline the synthesis of small molecules.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter