Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Cell-Wall Set Point
Closely related bacterial enzymes make cell-wall polymers of varying lengths
by Celia Henry Arnaud
October 20, 2008
| A version of this story appeared in
Volume 86, Issue 42

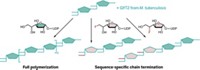
ISOLATED CELL-WALL ENZYMES from different bacteria make cell-wall polymers with different chain lengths, even under chemically identical conditions, Harvard University researchers report (J. Am. Chem. Soc., DOI: 10.1021/ja806016y). Elucidating this process could lead to a better understanding of cell-wall architecture and to improvements in other systems for making polymers.
Bacteria are encased in a cell wall made of cross-linked peptidoglycan strands. Enzymes called peptidoglycan glycosyltransferases (PGTs) string together a disaccharide that has a peptide and a lipid attached to one sugar to make these polymers. The glycan strands are then cross-linked through the attached peptides. The Harvard team, led by chemistry professor Daniel Kahne and microbiology and molecular genetics professor Suzanne Walker, used a radiolabeled version of this disaccharide to study the synthesis of these cell-wall polymers.
The researchers reasoned that they could use this radiolabeled analog to compare different PGTs because the enzymes are closely related in structure and the chemical compositions of the bacterial cell walls they make are identical in terms of glycan chain. Although different bacteria have cell walls of similar composition, bacterial shapes are quite different. That made Kahne wonder: "Do these individual enzymes make different-length polymers, or are there other factors in the cell that are responsible for tailoring these polymers to make the different shapes these bacteria have?"
Using the analog, graduate student Tsung-Shing Andrew Wang and undergrad Sara A. Manning found that not only do enzymes from different organisms make glycans of different lengths, but those lengths don't depend on the substrate concentration, even at an enzyme-to-substrate ratio of 1:100. "That's kind of weird," Walker says.
The polymer length seems to be a preprogrammed characteristic of each enzyme, suggesting that a termination and release mechanism is involved. Walker and Kahne have not yet figured out that mechanism.
At a 1:1 ratio of enzyme to substrate, which might be expected to yield single turnover products, the enzymes still make the longer glycans. "There's a slow initiation step, and then, obviously, polymerization is faster than the slow binding step," Kahne says. "Otherwise, when you have a 1:1 enzyme-to-substrate ratio, you would just get a single turnover and then run out of substrate."
Walker envisions using such a termination-and-release mechanism, once it's uncovered, to improve other systems for making polymers. "Controlling polymer length is something people want to be able to do," she says.
The study is also likely to interest microbiologists, who are keen to learn more about glycan synthesis and chain length because such information might shed light on cell-wall architecture and support one of the competing models of cell-wall assembly. "You would think, given that people have studied the bacterial cell wall for 50 years, that we would at least know whether it grows parallel or perpendicular to the cell surface," Kahne says. "Our work does not definitively rule out one of the two models."
Jean van Heijenoort, a researcher at the Institute of Molecular & Cellular Biochemistry & Biophysics at the University of Paris-South, in Orsay, France, cautions that these experiments only partly reflect the in vivo synthesis of cell-wall polymers. "The next step would be to analyze the glycan chain lengths of the products obtained when the transglycosylation and transpeptidation reactions are considered together in an in vitro experiment," he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter