Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Quinine Quest
Final disputed steps of the antimalarial alkaloid's first total synthesis confirmed
by Bethany Halford
February 4, 2008
| A version of this story appeared in
Volume 86, Issue 5
BY REVISITING a 90-year-old synthetic procedure, chemists Robert M. Williams and Aaron C. Smith have completed the latest—and possibly the last—chapter in the epic saga of the first total synthesis of quinine.
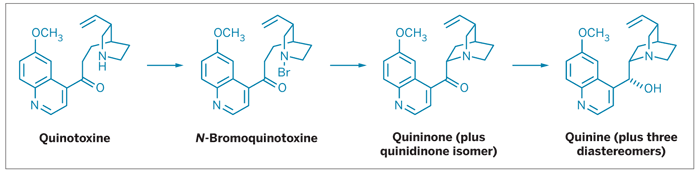
The story began in 1944, when Harvard University chemists R. B. Woodward and William von Eggers Doering published a communication on "The Total Synthesis of Quinine" (J. Am. Chem. Soc. 1944, 66, 849). The achievement garnered accolades from both the chemical community and the press, coming at a time when the Allied forces fighting in World War II had been cut off from their sole source of the antimalarial compound.
Although it was originally described as a total synthesis of quinine, chemists now recognize Woodward and Doering's work as a formal total synthesis—a relay-type effort in which one group synthesizes a compound that another group has already transformed into the final product. In 1944, Woodward and Doering actually prepared quinotoxine. The final three steps to transform quinotoxine to quinine had already been reported by German chemists Paul Rabe and Karl Kindler back in 1918 (Ber. Dtsch. Chem. Ges. 1918, 51, 466). Rather than repeat Rabe and Kindler's work, Woodward and Doering simply referenced the 1918 report, a common practice in natural product synthesis.
In the intervening years, however, some chemists, most notably Columbia University's Gilbert Stork, began to cast doubt upon Rabe and Kindler's work and, by extension, Woodward and Doering's synthesis.
"I am just astounded that no one had taken the trouble to repeat those last three steps, because it's actually very easy to do," says Williams, a chemistry professor at Colorado State University, in Fort Collins. So he and his postdoc Smith reproduced Rabe and Kindler's procedure and found that with some tinkering, they were able to get it to work (Angew. Chem. Int. Ed., DOI: 10.1002/anie.200705421).
The scheme's main hang-up is in the last step, where quininone is reduced to quinine with aluminum powder. Smith found that fresh aluminum powder gave only traces of quinine. Aluminum that had been exposed to air—which was likely the case with Rabe's aluminum in 1918—gave much better yields, indicating it's actually an Al(III) impurity that's responsible for the reduction.
"I think Williams and Smith did an excellent job of figuring out how to modify aluminum powder so they could reproduce the Rabe-Kindler portion of the Woodward-Doering route to quinine," Stork tells C&EN. "It's a shame that this wasn't done by Woodward and Doering."
"It was gratifying to see that the Williams group could reproduce this process," comments Steven M. Weinreb, a chemistry professor at Pennsylvania State University. "Hopefully, this nice piece of work will put the whole debate to rest."


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter