Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Anticancer Agent Created, Corrected
Two neopeltolide total syntheses lead to its structural reassignment
by Stu Borman
February 11, 2008
| A version of this story appeared in
Volume 86, Issue 6
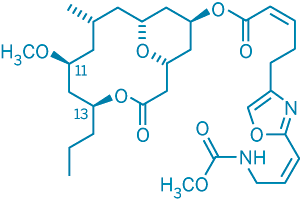
The correct structure of neopeltolide. In the structure proposed originally, the stereochemistry at positions 11 and 13 was reversed.
TWO RESEARCH GROUPS have found a good recipe for a synthetic organic study: identify a structurally intriguing and medically promising compound, invent a procedure for making it, and add a pinch of structural reassignment for good measure.
The two teams have just tested this recipe on neopeltolide, a recently discovered natural product that was isolated from a marine sponge and was found to have unusually potent anticancer properties. In the process of assembling the molecule from scratch, they also managed to correct its original, inaccurately assigned structure.
The studies were carried out independently by organic and medicinal chemist James S. Panek and coworkers Willmen Youngsaye, Jason T. Lowe, Frauke Pohlki, and Paul Ralifo at Boston University (Angew. Chem. Int. Ed. 2007, 46, 9211) and by bioorganic chemist Karl A. Scheidt and coworkers Daniel W. Custar and Thomas P. Zabawa at Northwestern University (J. Am. Chem. Soc. 2008, 130, 804). The work is an example of how organic synthesis can be useful not only for making molecules but also for resolving challenging structural problems.
"Synthesis makes it possible to make distinct stereochemical entities that would otherwise be inaccessible," Panek says. "It's not uncommon to need organic synthesis to come to the right conclusion" about the structure of a new chemical entity.
The neopeltolide saga began early last year, when marine natural products chemist Amy E. Wright and coworkers at Harbor Branch Oceanographic Institution, in Fort Pierce, Fla., reported the isolation, identification, and characterization of neopeltolide (J. Nat. Prod. 2007, 70, 412). The tantalizing compound was found in a marine sponge that one of Wright's colleagues, using a manned submersible, collected from a 1,450-foot-deep rocky outcrop in the ocean off northwest Jamaica.
In tests of the compound's biological activity, Wright and coworkers found the compound to be an unusually powerful antitumor agent, having potency about as strong as that of the commercial anticancer drug Taxol but with a different mechanism of action (cell cycle arrest instead of microtubule stabilization). It also had a challenging structure, so both Panek's and Scheidt's groups decided to try to synthesize it.
Wright and coworkers used advanced nuclear magnetic resonance techniques to determine neopeltolide's relative stereochemical configuration. But the Panek and Scheidt groups' synthetic efforts revealed that the structure proposed by Wright and coworkers was incorrect: The relative stereochemistry of two of the compound's carbon atoms had been reversed. Uncertainties in the data—a close similarity between NMR interatomic distance measurements in the correct and incorrect structures—had led to the inaccurate assignment.
Both synthetic groups first synthesized the compound with the incorrect structure and then noticed that its properties didn't match those of the natural product. Panek and coworkers narrowed down structural alternatives by evaluating NMR data; Scheidt's group carried out modeling studies to predict the actual structure. Both groups eventually synthesized the correct compound.
Neopeltolide has two main components—a macrolide with an embedded pyran ring and an oxazole-containing side chain—and the two teams devised complementary approaches to build and combine the components.
To form the pyran-containing macrolide, Panek and coworkers used an asymmetric dihydropyran annulation reaction they developed earlier. They then used an olefination reaction to add the side chain to the macrolide.
Scheidt and coworkers created a macrolide containing a pyranone (a pyran with an added ketone) by using a Lewis acid-catalyzed macrocyclization reaction they had developed earlier. They then used a Mitsunobu reaction to convert the pyranone to a pyran and simultaneously attach the side chain to the macrolide.
The discovery of neopeltolide's structurally incorrect assignment is but the latest in a series of similar cases in which organic synthesis has served as a key structural arbiter. For example, total syntheses of the marine metabolite palmerolide A reported independently last summer by two groups—one led by Jef K. DeBrabander of the University of Texas Southwestern Medical Center, in Dallas, and another headed by K. C. Nicolaou of Scripps Research Institute and the University of California, San Diego, and David Y.-K. Chen of the Agency for Science, Technology & Research, in Singapore—led to structural revision of that natural product.
With neopeltolide now available by two synthetic routes, efforts to synthesize analogs and test them for bioactivity have begun. Each of the total syntheses has its advantages, such as making specific types of analogs more easily accessible. In this case, therefore, two cooks definitely have not spoiled the broth.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter