Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Using Yeast To Make Alkaloids
Large part of benzylisoquinoline pathway reconstructed in yeast
by Carrie Arnold
August 13, 2008

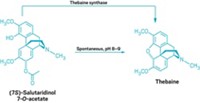
With help from plant and human enzymes, yeast (Saccharomyces cerevisiae) can now make certain pharmaceutically important alkaloids naturally produced by plants. Previous efforts to biosynthesize benzylisoquinoline alkaloids (BIAs) have involved several types of microbes, resulting in a complicated, inefficient process. The new technique, devised by Christina D. Smolke, assistant professor of chemical engineering at Caltech, and graduate student Kristy M. Hawkins, greatly simplifies this process and improves yields (Nat. Chem. Biol., DOI: 10.1038/nchembio.105).
BIAs include a variety of plant compounds, most notably the opiate morphine, the toxin sanguinarine, and their derivatives. Many BIAs are of economic importance as analgesics, dyes, and antibiotics. However, they generally must be extracted directly from plants, which produce only minute amounts of BIAs. The few BIA compounds that can be synthesized in organic chemistry labs typically are generated in dismally low yields. What’s more, researchers have thus far been unable to elucidate many of the plant enzymes involved in BIA synthesis.
Now, using genes that code for three enzymes in plants and for a cytochrome P450 enzyme from humans, Hawkins and Smolke engineered S. cerevisiae to transform (R,S)-norlaudanosoline, a commercially available substrate, into the BIA intermediate (R,S)-reticuline. Then the same engineered yeast converts the R-isomer of reticuline into morphine and the S-isomer into berberine (shown).
Smolke notes that the use of a human cytochrome P450 enzyme to convert reticuline into a metabolite of morphine was “a previously uncharacterized activity for this enzyme.”
Before Hawkins and Smolke’s most recent efforts, the biosynthesis of BIAs relied on sequential reactions in several different microbes. After each step, researchers would have to purify the desired intermediate and then feed it to the next set of microbes, yielding only small amounts of the final product.
Smolke noted that even though she and Hawkins haven’t yet optimized the yeast fermentation process, they have obtained “reasonably good” yields for BIAs of pharmaceutical interest. Through optimization Smolke hopes to increase yields 10- to 100-fold.
Toni Kutchan, a researcher at the Donald Danforth Plant Science Center and an adjunct professor of biology at Washington University, both in St. Louis, said this work on the biosynthesis of BIAs may “provide useful tools for gene function discovery and for microbial synthesis of novel alkaloids that could be tested as potential new drugs.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter