Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Mechanical Force Activates Catalyst
Ultrasound turns on silver- and ruthenium-based catalysts
by Rachel Petkewich
April 13, 2009
| A version of this story appeared in
Volume 87, Issue 15

MECHANICAL FORCE now joins light, heat, and chemicals on the short list of ways to activate a homogeneous catalyst.
Chemists are intrigued by the possibilities of the new mechanical approach, which was developed by chemistry professor Rint P. Sijbesma and coworkers at Eindhoven University of Technology, in the Netherlands. “Coupling catalyst activity to a mechanical force is in principle completely orthogonal to the use of light, heat, or chemical activation,” says Stephen L. Craig, a chemist at Duke University. One can imagine systems of multiple catalysts where one catalyst can be controlled specifically by force while others are controlled by more conventional stimuli, he says.
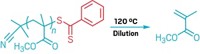
Sijbesma and postdocs Alessio Piermattei and Karthikeyan Sivasubramanian report two different metal-carbene catalysts that can be activated by mechanical force. They demonstrate that ultrasound-generated force triggers a silver-based catalyst to perform transesterification and a ruthenium-based catalyst to facilitate olefin metathesis (Nature Chem., DOI: 10.1038/nchem.167).
“We have shown in two distinct systems that a catalyst could be ‘switched on’ when one of the carbene ligands was pulled off from an inactive precursor metal complex,” Sijbesma says. “In each case, the mechanical force was transferred to the metal complex through poly(tetrahydrofuran) polymer chains attached to the ligands,” he adds. They note that without polymers on the ligand, no ultrasound-induced activity is observed. They also find that catalytic activity stops when ultrasound ceases because the catalyst degrades over time, and dissociated ligands do not rebind to regenerate a latent catalyst.

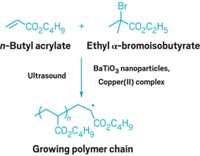
The mechanical force comes from the collapse of cavitation bubbles that form in solution during exposure to ultrasound. Applying ultrasound to accelerate reactions is not new, and researchers at the University of Illinois, Urbana-Champaign, have shown that mechanical energy can trigger stoichiometric ring-opening reactions (C&EN, March 26, 2007, page 9).
Although the yields of both of the small-molecule transformations achieved with these polymeric catalysts are no match for their respective nonmechanochemically activated parent catalysts, the team's mechanochemically activated version of the ring-opening methathesis polymerization (ROMP) reaction is promising, write Jitendra S. Rathore and Alshakim Nelson of IBM Almaden Research Center, in San Jose, Calif., in a related commentary. "ROMP reactions by a dormant catalyst hold significance in light of the recent interest in self-healing materials," they add.
Although the yields of both of the small-molecule transformations achieved with these polymeric catalysts are no match for their respective nonmechanochemically activated parent catalysts, the team’s mechanochemically activated version of the ring-opening methathesis polymerization (ROMP) reaction is promising, write Jitendra S. Rathore and Alshakim Nelson of IBM Almaden Research Center, in San Jose, Calif., in a related commentary. “ROMP reactions by a dormant catalyst hold significance in light of the recent interest in self-healing materials,” they add.
Although such applications have a way to go to become reality, the possibilities are exciting, Craig says. “Chemists are just beginning to think about using mechanical forces as productive tools for bond-forming chemistry,” he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter