Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Main-Group Research Springs Ahead
Chemists retool s- and p-block compounds in hopes of widening their use in reaction chemistry
by Stephen K. Ritter
April 20, 2009
| A version of this story appeared in
Volume 87, Issue 16
YELLOW AND BLUE make green. That color combination, familiar to children coloring with crayons, was not what chemist Richard A. Kemp was thinking when he first saw yellow and green crystals of a pure gallium diimine complex. It took a little detective work, and a determined undergraduate student, but Kemp's group figured out that the normally yellow compound appears green in places where a tiny amount of a virtually undetectable blue dimer of the compound hides within the crystals.

Welcome to the fascinating and sometimes surprising world of main-group chemistry.
Kemp is a chemistry professor at the University of New Mexico and a staff scientist in the Advanced Materials Laboratory at Sandia National Laboratories. He presented his intriguing tale of discovery during a Division of Inorganic Chemistry symposium focusing on main-group chemistry that he coorganized at the ACS national meeting held in Salt Lake City last month. Kemp was joined at the meeting by an international array of main-group chemists who presented their latest developments during several inorganic symposia that highlighted the broader role that main-group compounds are playing in catalysis.
Main-group compounds—those featuring elements in groups 1 and 2 and groups 13 to 18 of the periodic table, carbon excepted—can be difficult to synthesize and characterize, the outcomes often being unpredictable, Kemp told C&EN. That's true in particular for the less common elements. But that hasn't diminished main-group chemistry's leading role in developing functional materials, including ceramics, semiconductors, inorganic polymers, and sol-gel materials, he said.
"Materials research and development involving main-group chemistry no doubt will continue," Kemp noted. "However, a new trend that is becoming more apparent is an emphasis on using main-group elements as substitutes or alternatives to transition-metal catalysts and other types of complexes." In particular, using main-group metals to activate small molecules such as H2, CO2, and NH3 for organic chemistry and energy-related research themes will rival the use of transition-metal complexes, he predicted.
The symposium that Kemp coorganized with chemistry professor Michael Lattman of Southern Methodist University led the way in demonstrating the trend. It was dubbed "CowleyFest" as a tribute to chemistry professor Alan H. Cowley of the University of Texas, Austin, who received the 2009 ACS Award for Distinguished Service in the Advancement of Inorganic Chemistry (C&EN, Jan. 12, page 45).
Cowley, who delivered a retrospective award address at the meeting, is credited with shifting the emphasis in main-group chemistry from compounds built around elements in high oxidation states and with high coordination numbers in the 1960s to low oxidation states and low coordination numbers today. A lower oxidation state typically means more valence electrons are available for more diverse chemistry to take place.
LIKE THE CHEMISTRY described by many of the CowleyFest speakers, Kemp's chemistry bore the influence of Cowley's pioneering work. His multichromic crystal detective story began about 15 years ago, when he was a researcher at Union Carbide studying the reactions of diimines with aluminum and gallium alkyl-halogen complexes—work inspired by Cowley—to make olefin polymerization catalysts.
"It is immensely satisfying to see main-group chemistry continue to blossom."
One gallium aryl diimine complex formed single crystals that were all yellow, all green, half yellow and half green, or mostly yellow with some of the facets green, Kemp related. When separated, the different-colored crystals had the same nuclear magnetic resonance spectra, the same elemental analyses, and even the same single-crystal X-ray structures, he said. But the gallium compound was going nowhere as a catalyst precursor, so he had to drop the research thread and didn't have a chance to unravel the color conundrum.
Last year, Kemp picked up where he left off. His current group repeated the reaction of the aryl diimine with Ga(CH3)2Cl, which was forecast to form a Lewis acid-base complex, LGa(CH3)2Cl, where L is the diimine ligand. Instead, one methyl group gets shuffled around to take up residence on one of the diimine carbon atoms, forming CH3LGaCH3Cl—the compound with the yellow and green crystals.
It turns out that the green color arises from the presence of a tiny amount of an intensely blue dimer formed from the yellow principal compound, Kemp explained. As the dimer forms in the presence of excess Ga(CH3)2Cl, two molecules of H2 are eliminated. The hydrogen atoms are hardly missed and leave the parent compound and dimer virtually indistinguishable.
Undergraduate student Giang Nguyen discovered the blue dimer when she managed to tease it out of the product mix. The compound is probably dispersed throughout the crystals, Kemp said, but it selectively concentrates at certain facets of the growing crystals to give the green color. The dimer appears to be a biradical, which leads to the intense color, he added.
This particular gallium complex seems to be the only one that gives the mixed-color crystals. Kemp's group hasn't yet determined whether the multichromic crystals could serve some practical application, "but they sure are interesting," he mused.
Kemp's intriguing story was just one of many told at CowleyFest. Associate chemistry professor Charles L. B. Macdonald of the University of Windsor, in Ontario, has been working with indium, one row south of gallium in group 13 of the periodic table, to create better starting materials to carry out indium(I) chemistry.
Indium(III) is the most stable oxidation state of the element, Macdonald said, but In+ is more interesting because it has an extra pair of electrons. That pair of electrons makes a big difference: In3+ is a Lewis acid, whereas In+ can act as a Lewis base. As a Lewis base, In+ can function as an electron-donating ligand for main-group and transition-metal electron-acceptor species.
"The electron-rich nature of low-oxidation species like In+ often provides for fundamentally and drastically different structural features and reactivity patterns than those observed for the standard oxidation states," Macdonald told C&EN. "It's not surprising that the low-oxidation compounds tend to be less stable, thus the preparation and isolation strategies for these compounds typically require the use of steric and/or electronic stabilization by suitable covalently bonded ligand systems."
To that end, Macdonald's group has been working on an alternative way to isolate low-oxidation-state compounds, using indium as a test model. Developing In+ chemistry has been hampered by the insolubility and instability of indium halides, which are the natural choice for starting materials, Macdonald said. To overcome this obstacle, his group prepared indium triflate, InOSO2CF3. Unlike the In+ halides, InOSO2CF3 is relatively stable and unusually soluble in organic solvents. Macdonald's group synthesized it in several ways, including treating InCl with triflic acid, HOSO2CF3.
InOSO2CF3 is proving to be a useful reagent for making indium polyhedral clusters and cyclopentadienyl sandwich compounds, Macdonald reported. It is also serving well as a catalyst for organic C–C bond-forming reactions, he said. His team is now extending the strategy to make stable gallium(I) and aluminum(I) compounds, the halides of which can currently be prepared only in the gas phase.
Macdonald also reported that InOSO2CF3's stability and solubility can be boosted even further by coordinating indium with crown ether ligands. For example, ligating InOSO2CF3 with [18]crown-6 created the first stable monomeric inorganic In+ complex. The researchers subsequently showed that crown ethers that are too small to encapsulate the In+ cation, such as [15]crown-5, form sandwich complexes in which a "naked" In+ cation (stripped of OSO2CF3–) is held between two crown ether molecules.
The crown ether approach is amenable to other main-group metals and nonmetals. For example, Macdonald's group and the groups of collaborators Kim M. Baines and Paul J. Ragogna of the University of Western Ontario are investigating group 14 crown ether complexes that "have produced a multitude of unexpected and exciting results," Macdonald said. However, he is not able to divulge the details pending publication.
As a harbinger of what might be coming, though, Baines's group recently reported using a related cryptand macrocycle as a ligand to isolate a naked Ge2+ cation (C&EN, Dec. 1, 2008, page 12). That is the first time anyone has isolated a doubly charged nonmetallic ion without any covalent bonds.

SHIFTING EAST on the periodic table, Massachusetts Institute of Technology chemistry professor Christopher C. Cummins outlined his group's pursuit of transition-metal complexes that can be used in cyclic reactions to create novel main-group species directly from the pure elements.
One of Cummins' breakthroughs is a niobium phosphide complex that features a terminal Nb≡P bond. This complex, created by using white phosphorus, P4, has served as a workhorse transfer reagent in the Cummins lab to shuttle P2 and phosphorus-containing units such as EP2 rings (E = Ge, Sn, or Pb) to organic molecules or to transition-metal complexes.

The tetrahedral P4, one of phosphorus' stable elemental forms, is derived by reducing apatite, a phosphate mineral. P4 is used as a starting material to make phosphoric acid, phosphorus trichloride, and other commodity chemicals that are vital reagents for research and industrial syntheses.
Making new compounds directly from P4 and other "tetraatomic tetrahedra" rather than first making a commercial reagent is more efficient and greener chemistry, Cummins told C&EN. In that regard, the recyclable niobium phosphide complex is opening up "exciting possibilities for activating small molecules and developing reagents for metathesis or atom-transfer reactions," he said.
At the ACS meeting, Cummins recounted his group's efforts to expand the niobium-shuttling strategy to the heavier group 15 elements arsenic and antimony during a symposium in honor of his MIT colleague Daniel G. Nocera, recipient of the 2009 ACS Award in Inorganic Chemistry (C&EN, Feb. 23, page 66).
As a P4 analog, As4 is woefully unstable. But Cummins figured that AsP3 might serve just as well. Using a niobium complex with a terminal P3 ring ligand as the shuttle, the team converted AsCl3, into AsP3 (Science 2009, 323, 602). To characterize the compound, the researchers treated AsP3 with a molybdenum complex, which coordinated AsP3 to molybdenum through one of the phosphorus atoms.
The successful demonstration with AsP3 opened the door for Cummins' group to cut loose and use NbE3 shuttles (E = P or As) to make a variety of main-group tetraatomic tetrahedra, including As2P2, As3P, and SbP3. In preliminary studies, the group also generated As4 on the fly in the lab and used it like P4 to make and use Nb≡As and NbAs3 shuttles. Cummins envisions AsP3 and its analogs as useful reagents in organic synthesis and as exact-ratio sources of group 15 elements for making advanced materials.
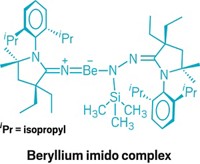
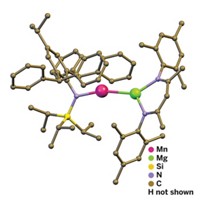
Moving to the far west of the periodic table, Cameron Jones of Monash University, in Victoria, Australia, used CowleyFest to recount his unprecedented work on creating magnesium(I) compounds and his early efforts to extend the chemistry to calcium(I) compounds. Until Jones came along, magnesium (and all group 2 metals) existed in the 2+ oxidation state in all its known stable compounds. But in late 2007, Jones and his Monash coworkers Andreas Stasch and Shaun P. Green reported the synthesis of RMgMgR complexes, where Mg is in the 1+ oxidation state and R is a diketiminate ligand (C&EN, Nov. 19, 2007, page 52).
THESE COMPOUNDS, made by reducing a Mg2+ iodide reagent, demonstrate several firsts: They are the first stable compounds containing Mg+ and the first compounds with Mg–Mg bonds. Metal-metal bonding has been a hot topic for years in transition-metal chemistry, but it has only recently made its way into main-group elements.
In Salt Lake City, Jones discussed new characterization and theoretical studies to support his initial thinking that the metal core of the Mg–Mg compounds can be regarded as a covalently bonded, two-center/two-electron Mg22+ unit stabilized by the bulky anionic ligands. The Mg–Mg bonds in the complexes can be remarkably elongated when treated with Lewis bases, such as ethers, to form stable adducts, Jones noted. In this respect, the Mg–Mg bonds appear to be "deformable," he said.
As for initial reaction chemistry, Jones said the Mg22+ compounds are proving to be versatile reducing agents for a variety of unsaturated organic, inorganic, and organometallic substrates. His team has shown that the compounds can facilitate C–C coupling, N–N coupling, oxidative insertions, and more (Angew. Chem. Int. Ed. 2009, 48, 2973). "These Mg22+ compounds could potentially serve as alternatives to sodium and potassium metal and samarium-based reducing agents, which are commonly used in organic and organometallic syntheses," he said.
Advertisement
After a whirlwind week of main-group chemistry, UT Austin's Cowley was impressed by what he views as a renaissance in the field. "Following what were fundamental studies years ago, many new materials developed from main-group compounds are now being used for practical purposes," Cowley told C&EN. "That progress is being mimicked today with fundamental research on catalysis, which is laying the base for new opportunities. It is immensely satisfying to see main-group chemistry continue to blossom."




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter