Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Facile Formation Of A Möbius Molecule
Researchers forge the first macrocycle to attain unassisted Möbius aromaticity; a Möbius antiaromatic compound could be next
by Bethany Halford
May 25, 2009
| A version of this story appeared in
Volume 87, Issue 21

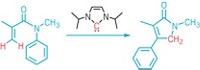
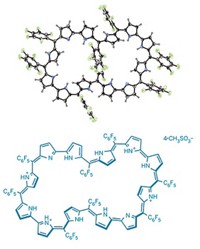
The challenge of making a molecule that contains the distinctive twist of a Möbius strip and is also aromatic in character has long appealed to organic chemists. Now, researchers have made the first macrocycle to attain Möbius aromaticity without any assistance from metal coordination, temperature control, or protonation (J. Am. Chem. Soc., DOI: 10.1021/ja902836x). Eliminating those constraints allows chemists to conduct detailed investigations of Möbius aromaticity that would otherwise be impossible, the researchers note. The team, led by Dongho Kim of South Korea's Yonsei University and Atsuhiro Osuka of Japan's Kyoto University, managed to juggle both the twist and the necessary girth for aromaticity by expanding a porphyrin ring. They made their Möbius molecule—a benzopyrane-fused [28]hexaphyrin—by simply heating a 26-membered hexaphyrin in acetic acid at 130 °C for six hours. This reaction fuses a perfluorophenyl substituent into the porphyrin structure, creating the tricyclic benzopyrane system that imparts the strain necessary to achieve the twisted Möbius topology. Kim and Osuka hope to use the information they've gleaned from this structure to create a Möbius molecule that is antiaromatic.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter