Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Ultrafast EELS
Extreme version of electron energy loss spectroscopy probes bonding dynamics
by Mitch Jacoby
July 13, 2009
| A version of this story appeared in
Volume 87, Issue 28
If a 10-fold increase in the resolving power of an analytical tool grabs the attention of scientists, imagine the response to a 10 billion-fold improvement. That is the magnitude of the time-resolution enhancement in electron energy loss spectroscopy (EELS) that researchers at California Institute of Technology reported last week (Science 2009, 325, 181).
In EELS experiments, researchers measure element-specific energy losses in an electron beam caused by interactions of the beam with atoms in a sample. The technique is often used in conjunction with transmission electron microscopy (TEM) to reveal the identity and the oxidation state of atoms in the nanometer-sized area probed by the TEM beam. Common EELS detectors respond on the millisecond timescale, which provides static pictures of samples either before or after chemical bonding changes occur (C&EN, July 6, page 10).
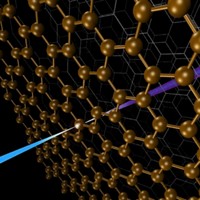
Now, Fabrizio Carbone, Oh-Hoon Kwon, and chemistry Nobel Laureate Ahmed H. Zewail have developed laser-driven TEM-based procedures for measuring EELS on a timescale that's faster by 10 orders of magnitude. The team demonstrated that the methodology can be used to map changes in chemical bonding with extreme time and spatial resolution, thereby providing a method for spying on those dynamic processes as they unfold.
To demonstrate the new method's resolving power, Zewail and coworkers stressed a thin graphite crystal with an intense femtosecond IR laser pulse. Then they recorded time-resolved EELS, electron diffraction data, and images that reveal that the laser pulse causes the spacing between the crystal's atomic layers to compress and expand on the picometer (10–12 meter) scale. The spacing changes mark momentary transitions between graphite (expanded) and diamond (compressed) structures. The data also provide snapshots that trace the evolution of the interatomic electron density distribution as the lattice spacings change. Specifically, the method records the evolution of electronic structure from sp2 hybridization characteristic of graphite to sp3 hybridization in diamond.
"The technical virtuosity in this study is dazzling," exclaims Cambridge University professor Sir John M. Thomas, a TEM and EELS expert. This advance "opens up a whole new world" in which scientists will be able to study the dynamics of chemical bonding in ways that have not been possible until now, he says. This coupled analytical tool, which probes matter with extreme time and spatial resolution, can be applied to a variety of systems, including cells, proteins, and solid-state materials, he adds.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter