Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Emergency Drug Fills Vaccine Void
Pandemic: FDA allows use of unapproved flu drug while vaccine supplies lag
by Ann M. Thayer
November 2, 2009
| A version of this story appeared in
Volume 87, Issue 44
Believing that the possible benefit outweighs any risk, FDA is allowing BioCryst Pharmaceuticals’ unapproved drug peramivir to be used in treating hospitalized flu patients. The new antiviral agent has been made available to battle the novel H1N1 influenza virus at a time when vaccines are overdue.
Just about a week ago, FDA issued an emergency use authorization for peramivir, following a request from the Centers for Disease Control & Prevention (CDC). With no adequate approved alternative, FDA says, the drug is meant for patients who aren’t responding to or can’t take Roche’s oral drug Tamiflu or GlaxoSmithKline’s inhaled product Relenza.
Discovered by Birmingham, Ala.-based BioCryst, peramivir inhibits neuraminidase, an enzyme that is critical to the replication of the flu virus. In the U.S., the company has the drug in Phase III clinical trials, which are being supported by $180 million in Department of Health & Human Services (HHS) funding. BioCryst’s partner, Shionogi, has completed late-stage studies in Japan and is filing for approval there.
“BioCryst has worked with HHS to enable the government to rapidly deploy an initial supply of peramivir, and we are prepared to deliver more,” says the firm’s CEO, Jon P. Stonehouse. The company already has provided HHS with 1,200 courses of treatment, each consisting of 600 mg per day for five days.
In preparation for orders from the U.S. or other governments, BioCryst is completing production of about 130,000 more treatment courses. According to analysts at the investment firm Leerink Swann, this could translate into about $39 million in sales for the company. Under the emergency use authorization, BioCryst is not allowed to promote its drug. CDC will control how and where peramivir is distributed.
Meanwhile, producers of H1N1 vaccines continue to have difficulty supplying as many doses as they once promised. As of Oct. 28, about 23.2 million doses were available, HHS Secretary Kathleen Sebelius said in a press briefing. Although the number of doses grew by about 9 million in one week, it is much below the 45 million doses that were anticipated to be on hand by mid-October and the total of 250 million doses reserved under contracts.


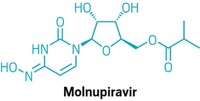

Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter