Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Flu Enzyme's Thieving Ways
X-ray crystal structures explain how influenza's RNA polymerase helps conquer human cells
by Carmen Drahl
February 9, 2009
| A version of this story appeared in
Volume 87, Issue 6

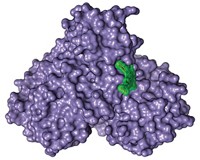
Structural detective work has fingered a protein thief that precipitates flu infections, an advance that could lead to new flu drugs. Influenza viruses use their RNA polymerase enzymes to steal short tags from the host's RNA. They then use those tags to commandeer the host's protein synthesis machinery for making viral proteins. However, researchers don't understand how each part of the polymerase contributes to the operation. Now, two independent teams have discovered that a portion of a polymerase subunit called PA acts as an endonuclease, which is the enzyme responsible for snipping the tags off the host's RNA. Stephen Cusack, Rob W. H. Ruigrok, and coworkers at the European Molecular Biology Laboratory and the University of Grenoble, both in France, identified the culprit when they solved the X-ray crystal structure of PA from a seasonal flu virus (Nature, DOI: 10.1038/nature07745). Zihe Rao of Tsinghua University, Yingfang Liu of the Chinese Academy of Sciences, both in Beijing, and colleagues reached the same conclusion, examining the corresponding portion of avian flu (Nature, DOI: 10.1038/nature07720).


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter