Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Self-Assembled Nucleotide Helix
Structure may have implications for prebiotic chemistry
by Stuart A. Borman
February 16, 2009
| A version of this story appeared in
Volume 87, Issue 7

A CHEMICAL STRUCTURE that has been sought for nearly 50 years—that of self-assembled 5′-guanosine monophosphate (5′-GMP)—has just been determined. The structure suggests how nucleotide monomers might line up to form covalently bonded nucleic acid oligomers and thus has potential implications for prebiotic chemistry.
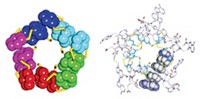
In 1962, David R. Davies and coworkers at the National Institutes of Health studied GMP assemblies. Their X-ray diffraction data showed that the nucleotides form G-quartets—planar sets of four hydrogen-bonded nucleotides—and suggested that the overall assemblies are helical. But the exact structure of these assemblies has remained unknown, despite efforts over the years by several groups to obtain it.
Now, chemistry professor Gang Wu and grad student Irene C. M. Kwan of Queen's University, in Kingston, Ontario, have determined the nuclear magnetic resonance structure of the assemblies in a solution containing sodium ions (J. Am. Chem. Soc., DOI: 10.1021/ja809258y).
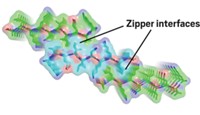
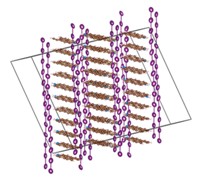
The structure shows that G-quartets stack to form a four-stranded helix, with each quartet offset from the next by a twist angle of 30º. Sodium ions run along the helix's central axis. The quartets are linked to one another not by phosphodiester bonds, as in RNA and DNA oligomers, but by weak molecular interactions, such as hydrogen bonds and ion-dipole forces.
Wu speculates that the structure couldn't be determined earlier because the assembly is hard to crystallize, its NMR spectrum is highly overlapped and hard to interpret, and its noncovalent linkages make it difficult to model theoretically. His group used sophisticated NMR techniques to overcome these problems.
The work provides a "convincing and probably definitive structure elucidation," says chemistry professor Christian Detellier of the University of Ottawa, who has pursued the structure. "This finding opens the way to a variety of studies, including the role of cations in the self-assembly process and potential functions associated with this assembly. For example, would such a supramolecular structure have played a role in prebiotic chemistry?" he asks.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter