Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Ubiquitin Unfolds Doomed Proteins
Besides tagging proteins for destruction, ubiquitin also assists in the process by helping unfold the proteins
by Sophie L. Rovner
January 11, 2010
| A version of this story appeared in
Volume 88, Issue 2
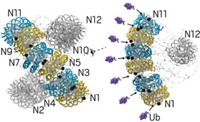
Unneeded or damaged proteins that are destined for destruction within a cell are tagged with ubiquitin. This polypeptide marks a condemned protein for degradation in a proteasome, a large protein complex otherwise known as the cell’s garbage disposal. Using computational techniques, Yaakov Levy and Tzachi Hagai of Israel’s Weizmann Institute of Science have discovered that ubiquitin also assists the degradation process by altering the protein’s thermal stability and thereby helping unfold the protein near the ubiquitin-binding site (Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.0912335107). The study “implies that, in addition to its known role as a recognition signal, the ubiquitin attachment may be directly involved in the cellular process it regulates by changing the biophysical properties of the substrate,” Levy and Hagai write. The researchers acknowledge that it is presently unclear how universal this mechanism is and to what extent nature exploits it in facilitating protein degradation. They add that ubiquitin’s effect on protein folding might be relevant to other cellular processes regulated by the polypeptide, including DNA repair and endocytosis.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter