Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Hydrogen Bond Reformulation
Proposed new definition broadens the qualifying chemical associations
by Jyllian N. Kemsley
November 22, 2010
| A version of this story appeared in
Volume 88, Issue 47

Textbooks currently define a hydrogen bond as a weak electrostatic interaction that forms between a lone pair of electrons on an electronegative atom and a hydrogen atom bonded to another strongly electronegative atom, typically oxygen, nitrogen, or fluorine. But that definition belies a rich assortment of chemical associations that have come to be called hydrogen bonds in the 90 years since Wendell M. Latimer and Worth H. Rodebush first coined the term (J. Am. Chem. Soc., DOI: 10.1021/ja01452a015). To address the disparity between definition and practice, a task group of the International Union of Pure & Applied Chemistry (IUPAC) has proposed a new, broader definition of hydrogen bonds.
The proposed definition is intentionally expansive: “The hydrogen bond is an attractive interaction between a hydrogen atom from a molecule or a molecular fragment X–H in which X is more electronegative than H, and an atom or a group of atoms in the same or a different molecule, in which there is evidence of bond formation.” The proposal, available at media.iupac.org/reports/ provisional/abstract11/arunan_310311.html, goes on to outline experimental and theoretical criteria that can be used as evidence for the presence of a hydrogen bond.
Difficulties in defining the hydrogen bond go back almost as far as the term, notes IUPAC task group chairman Elangannan Arunan, a chemistry professor at the Indian Institute of Science. In Linus Pauling’s 1939 book “The Nature of the Chemical Bond,” Pauling wrote that the energy of most hydrogen bonds is 2–10 kcal/mol—even as he gave an example, [F···H···F]–, that lies outside the range.
In 1960, responding to experimental evidence that weakly electronegative atoms and even π electron clouds could be hydrogen bond receptors (species with which H makes hydrogen bonds), George C. Pimentel and Aubrey L. McClellan provided a new definition in their book “The Hydrogen Bond.” Hydrogen bonds are “said to exist when 1) there is evidence of a bond, and 2) there is evidence that this bond specifically involves a hydrogen atom already bonded to another atom,” Pimentel and McClellan wrote.
By 1994, however, the pendulum of hydrogen bond inclusion had swung back the other way, and IUPAC published its current definition: “The hydrogen bond is a form of association between an electronegative atom and a hydrogen atom attached to a second, relatively electronegative atom. It is best considered as an electrostatic interaction, heightened by the small size of hydrogen, which permits proximity of the interacting dipoles or charges. Both electronegative atoms are usually (but not necessarily) from the first row of the periodic table, i.e., N, O, or F. Hydrogen bonds may be intermolecular or intramolecular. With a few exceptions, usually involving fluorine, the associated energies are less than 20–25 kJ mol–1 (5–6 kcal mol–1)” (Pure Appl. Chem., DOI: 10.1351/pac199466051077).
That definition represents a narrow view of hydrogen bonding that is not in line with scientific evidence, Arunan says. His interest in how the hydrogen bond is defined was piqued in 2004, when he and colleagues submitted a paper to Chemical Physics Letters about the interaction—which Arunan believes is a hydrogen bond—between C2H4 and H2S in the gas phase (Chem. Phys. Lett., DOI: 10.1016/j.cplett.2004.06.015). The reviewers all liked the paper, Arunan says, “but they said don’t call it a hydrogen bond.” Not wanting to pick a fight, Arunan and coworkers changed the paper to call their observation a bridging interaction between hydrogen bonding and van der Waals interactions.
But the experience made Arunan curious about the history of the definition and how thinking about it has evolved over time. At a subsequent IUPAC meeting in Bangalore, India, he asked whether IUPAC would be willing to delve into the definition again. He then formed a 14-member international task group. An account of the task group’s work will appear in an upcoming issue of Pure & Applied Chemistry; a separate essay on the group’s work was published last month by Gautam R. Desiraju, a chemistry professor at the Indian Institute of Science (Angew. Chem. Int. Ed., DOI: 10.1002/anie.201002960).
The group had many things to consider, including which elements can be involved in hydrogen bonding, the forces at play, and the nature of a system under study.
In the revised definition the group has proposed, atoms covalently bonded to hydrogen (the X in X–H) only have to be more electronegative than hydrogen. On the receptor side, the entire periodic table is in play. Hydrogen-bonding interactions involving transition metals such as platinum and the noble gases krypton and xenon have been noted. Carbon can be a receptor, at least in the form of CO or CH3 ∂. The hydrogen of a metal hydride can even be a hydrogen bond acceptor, in an interaction that is known as a dihydrogen bond.
As for the forces involved in a hydrogen bond, electrostatic interactions are key, but hydrogen bonds also have a covalent component, as evidenced by the fact that some electron density from the hydrogen bond receptor goes into an X–H antibonding orbital, changing the X–H stretching frequency—something that wouldn’t happen with a purely electrostatic interaction. Overall, the balance of forces that make up a hydrogen bond include electrostatic interactions, polarization, dispersion, charge transfer, and short-range repulsive effects from electron orbital overlap.
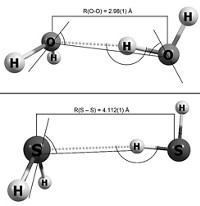
And whether a hydrogen bond exists may depend on experimental conditions. The crystal structure of solid H2S at –60 °C, for example, does not show any hydrogen bonding interactions. Cool the H2S down further and pressurize it, however, and it starts to behave like solid H2O.
After considering these and other issues, the task group arrived at the proposed definition. The criteria and characteristics in the definition do not all need to be satisfied, nor are they meant to be exclusive lists, Arunan says. Rather, they are intended to be a guide to researchers as they attempt to delineate the differences between covalent bonds, hydrogen bonds, and van der Waals forces.
“I applaud the attempt to put the controversies surrounding unconventional H-bonds to rest,” says Mark Mascal, a chemistry professor at the University of California, Davis, who was not part of the task group. But some linguistic kinks remain. For example, Mascal points to the use in the definition of “fragment,” which in mass spectrometry means a part of a molecule that has fragmented. He suggests that “constituent” or “component” might have been a better word choice. He also notes that confusion might arise from the common use of “X” to delineate a halogen; Mascal’s preference is to use “A–H.”
The task group, through Arunan, will be accepting comments on the proposed definition until March 31, 2011. Even if the final definition satisfies everyone, however, the discussion may not be over. Chemistry is an experimental science, and researchers will undoubtedly uncover new facets of the hydrogen bond in the years to come, says Steve Scheiner, a chemistry professor at Utah State University and cochair of the task group. “I wouldn’t be surprised if this definition has to undergo another review in 30 to 40 years, maybe sooner,” Scheiner says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter