Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Chlorination Improves Organic Electronics
Materials: Treatment could simplify manufacturing, reduce costs
by Jyllian N. Kemsley
April 18, 2011
| A version of this story appeared in
Volume 89, Issue 16

Chlorinating a common electrode material for organic light-emitting diodes (OLEDs) could make devices easier and less expensive to manufacture, researchers report (Science, DOI: 10.1126/science.1202992).
In a typical OLED, electrons move from an organic, light-emitting material to indium tin oxide. But the energy of the electrons removed from the light-emitting material is higher than the oxide can accept. Consequently, layers of other materials—for example, copper phthalocyanine—are used to bridge the gap and facilitate electron flow. The additional layers, however, add cost and complexity to manufacturing and reduce the electrical efficiency of electronic devices.
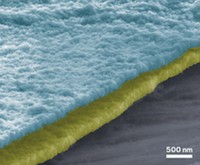
In an effort to do without those extra layers, a research group led by materials science and engineering graduate students Michael G. Helander and Zhibin Wang and professor Zhenghong Lu at the University of Toronto chlorinated the electrodes by exposing the material to o-dichlorobenzene and ultraviolet light. The treatment causes chlorine radicals from the solvent to displace oxygen and bind to indium on the electrode surface.
The resulting layer of polar In–Cl bonds increases the electrostatic potential just above the electrode’s surface. That change in potential increases the electron energy that the electrode can accept and closes the energy gap between the electrode and light-emitting materials, such as a phosphorescent iridium complex doped into 4,4´-N,N´-dicarbazole biphenyl. Electrons can then directly transfer between the light-emitting layer and the chlorinated electrode, making electronic devices easier to manufacture and more efficient to operate.
The prototype devices made by the Toronto group show a substantial improvement in operating voltages and efficiency, says Franky So, a materials science and engineering professor at the University of Florida. “This approach might lead to a paradigm shift in OLED technology.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter