Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
CO2 Helps Crystals Change Shape
Pharmaceutical Chemistry: Polymorphs convert under mild conditions
by Celia Henry Arnaud
January 17, 2011
| A version of this story appeared in
Volume 89, Issue 3
Exposure to elevated pressures of carbon dioxide can easily convert drugs from one crystalline form to another, chemists at the University of Missouri, Columbia, report (J. Am. Chem. Soc., DOI: 10.1021/ja107617m). The new method could have advantages over other techniques used to carry out such transformations in pharmaceutical manufacturing.
Many drugs exist in multiple crystalline forms, also known as polymorphs. “Controlling crystal forms is an absolutely key issue in the pharmaceutical industry,” says Jerry L. Atwood, a chemistry professor at the University of Missouri who led the research. A drug’s crystal form can dictate its solubility, stability, and compatibility with other components in a formulation, he notes.
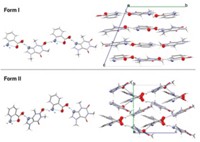
Obtaining the specific polymorph needed for a commercial product can be a long, arduous task. For example, the commercial form of the antibiotic clarithromycin is usually obtained by desolvating a “form 0” polymorph to yield “form I” and then heating form I for 18 hours at about 110 °C to convert it to “form II.”
Atwood, grad student Jian Tian, and postdoc Scott J. Dalgarno now achieve the same goal by pressurizing clarithromycin crystals with CO2. At a CO2 pressure of 350 psi, form 0 converts directly into form II, without generating form I first. The process takes only four hours at room temperature.
They also used CO2 under pressure to convert the ethanol hydrate of the drug lansoprazole to the solvent-free form used commercially. Desolvating the ethanol hydrate by vacuum drying is difficult because the drug readily decomposes.
So far, Atwood doesn’t know how CO2 is working its magic or whether it will work on all drugs. His “operating hypothesis” is that CO2 finds its way into small pockets in the crystal and “lubricates” the crystal lattice. “The pressures are far below what would be required for supercritical CO2,” he says. “We’re not dissolving and recrystallizing” the drug.
The technique “could be of great value in the study of the polymorphic space of organic compounds,” says Harry G. Brittain, head of the Center for Pharmaceutical Physics, in Milford, N.J. “This paper represents an exciting entry into a field that many thought to be mature.”




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter