Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Solar Power
Electrofuels Bump Up Solar Efficiency
Budding technologies provide nonphotosynthetic pathways to convert sunlight, carbon dioxide, and water into fuels
by Stephen K. Ritter
November 28, 2011
| A version of this story appeared in
Volume 89, Issue 48

Photosynthesis is nature’s way of storing energy from the sun in the form of electrons in chemical bonds. Humankind has long taken advantage of this process by using fossil fuels and biomass to produce heat, transportation fuels, and electricity. But photosynthesis is not efficient at converting sunlight into usable energy, and future global energy demand is expected to outstrip nature’s ability to provide the fuels we have grown to depend on.
Scientists and engineers are seeking ways to improve on photosynthesis, and some of them convened in Providence, R.I., earlier this month at the Society for Biological Engineering’s inaugural conference on electrofuels research to discuss their progress. Specifically, the conference focused on projects funded by the electrofuels program of the Department of Energy’s Advanced Research Projects Agency-Energy (ARPA-E).
Electrofuels are made with energy from the sun and renewable inorganic feedstocks such as carbon dioxide and water in processes facilitated by nonphotosynthetic microorganisms or Earth-abundant metal catalysts.
“The electrofuels concept is an effort to decouple the production of liquid fuels from fossil fuels and land use, which is starting to constrain our daily lives,” said Gregory Stephanopoulos, a chemical engineering professor at Massachusetts Institute of Technology and leader of the conference’s organizing committee.
“The name of the game for electrofuels is to find more efficient ways to capture solar radiation in a form that’s usable for transportation,” commented Eric J. Toone, a chemistry professor at Duke University and deputy director of ARPA-E. The agency was created in 2009 with funding from the American Recovery & Reinvestment Act to boost development and commercialization of technologies that reduce U.S. dependence on foreign energy sources.
“When looking at the amount of solar energy that is captured by a plant and the net amount of that energy it can harness, the value is on the order of 0.1 to 0.2%,” Toone said. “On the other hand, we know we can capture and convert solar energy to electricity at higher efficiency, on the order of 20% for current silicon photovoltaic cells. In the electrofuels approach, we’ve tried to bring together the best of both worlds, to develop catalytic systems capable of highly efficient energy assimilation from inorganic molecules for the direct production of fuels.”
At the electrofuels conference, MIT’s Daniel G. Nocera, one of the keynote speakers, described his group’s effort in developing electrolysis systems that use inexpensive metal catalysts in place of precious-metal catalysts such as platinum. The goal is to use only sunlight and water to make H2 to power fuel cells.
Nocera’s team formulated a cobalt borate photocatalyst for absorbing sunlight and splitting water to make H+ and O2. The catalyst closely mimics the properties of photosynthesis’s oxygen-evolving complex, including possessing self-healing properties that stabilize and extend the lifetime of the catalyst (C&EN, July 5, 2010, page 26). Nocera’s group also developed an efficient nickel-molybdenum-zinc alloy photocatalyst to couple H+ ions to form H2 (Science, DOI: 10.1126/science.1209816).
With the two catalysts, Nocera’s MIT group, working with Steven Y. Reece and Thomas D. Jarvi and coworkers at Sun Catalytix, a company Nocera founded, created an “artificial leaf.” The wireless solar-cell device mimics the ability of a natural leaf to convert sunlight into storable energy, Nocera explained. But it’s about 10 times more efficient than photosynthesis, he said.
Simply drop the solar cell in water and expose it to light, and O2 bubbles begin streaming off the side coated with the cobalt catalyst. At the same time, H2 bubbles begin streaming off the other side coated by the trimetal catalyst. If the solar cell is placed in a vessel with a barrier, the H2 and O2 could be collected separately, stored, and then later used to power a fuel cell, Nocera said.

Nocera envisions that a simple, low-cost, easily manufactured electrolysis system based on his catalysts would be the most useful way to supply power in regions of the world that lack electric-grid infrastructure. The system could also provide uninterrupted power for applications such as cell phone towers.
“The additional energy we will need in the future to maintain the status quo on our planet is for the 3 billion people now living in developing countries and the 3 billion people not yet born,” Nocera observed. “That’s our motivation for developing low-cost energy systems.” He likes to think of the future energy supply and demand as “fast-food energy.”
A solar cell based on Sun Catalytix’ design—about the size of a door—and 3 gal of water could produce enough electricity per day for the average U.S. home, Nocera noted. About a quart of water is all that might be needed in the developing world, he added. “I would be disappointed if we can’t get this off the ground and running within four years,” he said.
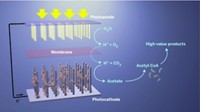
Other technologies presented in Providence relied on engineering microbes to get around photosynthesis’s inefficiencies. For example, Stephanopoulos’ group at MIT has an electrofuels project under way that taps two customized microbes: One produces acetate from waste CO2 and H2 from sunlight-driven water electrolysis and the second microbe converts the acetate into a triglyceride that can be processed into a fatty acid methyl ester, which is the primary component of biodiesel.
Reporting on another technology, microbiologist Derek R. Lovley of the University of Massachusetts, Amherst, described a lab-scale electromicrobiology approach to electrofuels that takes advantage of some microbes’ ability to make electrical contacts with materials. His team is growing Geobacter or Clostridium bacteria on iron(III) oxide films on carbon electrodes. The microbes consume electrons released from the electrode as their energy source and metabolize CO2. Differently engineered bacteria can produce compounds such as acetic acid or butanol.
The biofilms are stable and can be used in a continuous process, Lovley said. Even if the bacteria die or dry out, the biofilm is still active, he noted, because the activity is based on the electrical connections and not cell metabolism. “That’s a real strength of this system,” Lovley said. He next plans to work on a modular design to facilitate scale-up.
R. Mark Worden of Michigan State University reported his foray into designing bioreactors for growing microbes that can produce electrofuels. He’s drawing on the pharmaceutical industry’s experience with using large bioreactors to make antibiotics.
A number of parameters must be hammered out to produce electrofuels by microbial fermentation. These include the rate of uptake of feedstock gases by microorganisms, the solubility of gases in the fermentation medium, optimal reactor volume as large as 1 million L, and the safety of combustible gases. In working with H2, Worden said, “we would especially like to avoid having a ‘Hindenburg moment.’ ”
Worden is exploring increasing reaction surface area in large reactors by generating micrometer-sized bubbles stabilized with surfactants and electrolytes, which can help keep gases dispersed. Another idea, adopted from wastewater treatment, is to use reactor modules containing thousands of flow-through hollow fibers instead of large tank reactors.
As many modules as are needed can be linked together, he explained. The potentially explosive combination of H2 and O2 can be avoided by separating the gases in different fiber channels and allowing them to permeate into a microbial biofilm embedded in the fibers, where the chemistry would take place. Worden thinks a collaborative industry effort to develop such reactors makes sense, an idea he plans to pursue with the nonprofit Michigan Biotechnology Institute.
The technologies reported in Providence are the kind of high-risk, high-reward research ARPA-E was designed to fund, Toone told C&EN. The agency effectively acts as a venture capital agent for the federal government to step in where technology investors are shying away, he noted.
“You know going in that there is a long time horizon to commercializing these technologies, and they will cost a lot of money,” Toone said. “We know some technologies and some companies will fail. But because we have invested in these companies, we want to help the viable ones survive long term, which might mean they first produce intermediate products. The important point is developing the platform technology and proving it can work. The technology can later be adapted to meet market needs, be it a value-added chemical, commodity chemical, or a biofuel.”
For example, OPX Biotechnologies of Boulder, Colo., is developing an engineered-microbe technology platform based on hydrogenase enzymes produced by the bacterium Ralstonia eutropha. The ultimate plan, noted cofounder and Chief Scientific Officer Michael D. Lynch, is to produce biodiesel-equivalent fuel from CO2 and H2. But the company’s first commercial product, being developed with Dow Chemical, is going to be acrylic acid, a chemical used in paints, adhesives, diapers, and detergents.
OPX Bio’s engineered microbe makes 3-hydroxypropionic acid, which is then dehydrated to form acrylic acid through a Dow process. The method produces acrylic acid at 25% lower cost and with 75% less greenhouse gas emissions than petroleum-derived acrylic acid, Lynch said.
“Now, nearly two years into the electrofuels program, we are starting to see very clearly the contours of several approaches that look like they have legs and could be successful,” Toone said. “The question in front of us now, which is the much more difficult question, is that we know the electrofuels concept works, but is it going to matter, in the sense that can we take a technology and produce fuels at the scales that will be needed? This conference is the kickoff for answering that question.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter