Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Diagnostics
Lights and Lasers Invade the Clinic
A variety of optical techniques are slowly making their way into noninvasive medical diagnostics
by Aaron A. Rowe
January 2, 2012
| A version of this story appeared in
Volume 90, Issue 1

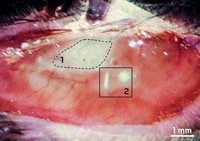
While zipping down a bike path along the beach in San Diego last August, Bruce J. Tromberg saw a huge pole directly ahead of him. He swerved to the left, but it was too late. He slammed his shoulder into the pole first, and then hit his chest. The injured chemist headed home, to Irvine, where he went to an emergency room. His arm was badly bruised, two of his ribs were broken, and his lung had collapsed. To make matters worse, he was scheduled to travel to an optics meeting across the country the following week.


Instead of attending the meeting, he headed for the Beckman Laser Institute & Medical Clinic at the University of California, Irvine, where he serves as the director. Tromberg told a pair of his graduate students to snap some photos of his wounds with an advanced imaging system that they had developed. Cobbled together from a digital camera and parts much like what you would find inside a liquid-crystal-display projector, it was able to show exactly where his badly bruised tissues were saturated with hemoglobin, revealing subsurface damage that isn’t visible to the naked eye. That technique is called spatial frequency domain imaging, and it is being commercialized by Modulated Imaging, one of the four optical diagnostics start-ups that are being incubated within the Beckman Laser Institute.
The instrument that Tromberg used to get a better view of his injuries uses just one of myriad new optical techniques that are in the early stages of clinical validation. Backers of such light-based medical diagnostics argue that these fast and typically noninvasive tools could someday surpass other imaging and in vitro methods.
The researchers who are developing these new techniques hope that they will see the same level of success that has been achieved by optical coherence tomography. That technique “is absolutely exploding in clinical practice as one of the best high-resolution methodologies to ‘see’ what is going on in the retina, a structure that is difficult to image,” says Bryan W. Jones, a neuroscience researcher at the University of Utah’s John A. Moran Eye Center.
Some of the most promising new optical techniques are multiphoton microscopy, second harmonic imaging, photoacoustic tomography, nonlinear Raman-based methods, and diffuse optical spectroscopy.
At the Beckman Laser Institute, physics graduate students are tinkering with a diffuse optical spectroscopy instrument in one room, while doctors examine patients in the next room. Diffuse optical spectroscopy allows researchers to look deep into the body. It turns the body into a cuvette, Tromberg explains. For instance, a beam of near-infrared light can shine through the skull and then scatter back out, painlessly giving information about how much oxygenated and deoxygenated hemoglobin are within the brain’s blood vessels. That data could be used to figure out whether a patient is bleeding internally from a stroke, suffering from another type of neurovascular damage, or responding to a drug regimen that affects the brain. A similar device, developed by Philadelphia-based diagnostics firm InfraScan, was approved in December 2011 by the Food & Drug Administration for the diagnosis of intracranial bleeding caused by head injuries.
In one such study, researchers at the Center for Earth Science Studies, in India, were able to diagnose several forms of oral cancer in a cohort of 96 patients (BMJ Open, DOI: 10.1136/bmjopen-2011-000071).
Using several different instruments, the smallest of which is about the size of a hefty hardcover novel, researchers at the Beckman Laser Institute can painlessly monitor a woman’s response to chemotherapy for breast cancer. More recently, they have started using those same instruments to study the connection between lipid function and blood flow in metabolic disorders.
Meanwhile, Infraredx, a Massachusetts-based company, has developed a diffuse optical spectroscopy instrument that relies on a fiber-optic probe that can be threaded into blood vessels. The device can collect both ultrasound images and diffuse reflectance spectroscopy data, which cardiologists can use to pinpoint lipid core plaques within blood vessels (J. Am. Coll. Cardiol. Img., DOI: 10.1016/j.jcmg.2008.06.001). Because the optic probe operates at near-infrared wavelengths, it can ‘see’ straight through blood, a problem that confounds other intravascular imaging methods.
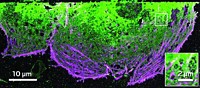
On the other side of a curtain from the diffuse optical spectroscopy equipment, Mihaela Balu, a postdoc at Beckman Laser Institute, is taking photos of live skin cells within her own arm with a JenLab microscope. It combines multiphoton spectroscopy and second harmonic generation to create images of live tissue within the human body. It illuminates natural fluorophores within those tissues, such as NADH or collagen, to create contrast. This could allow dermatologists to look for skin cancer without doing a biopsy, for example.
Beyond such natural fluorophores, Tromberg is excited about the many nanoparticles and small molecules that could be used as labels for multiphoton microscopy. There has been an explosion of research in that area, he says. However, such synthetic contrast agents require regulatory approval, which can take years.
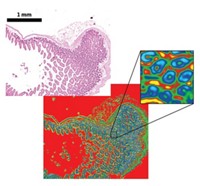
For vivid three-dimensional images of deep tissue, researchers are turning to photoacoustic tomography techniques. In these methods, a carefully tuned laser excites specific bonds so aggressively that they make a little bit of noise. Passive acoustic systems then listen for those snaps, crackles, and pops to create an image. For instance, Ji-Xin Cheng and his colleagues at Purdue University used light at 1,730 nm to rattle the methylene groups of lipids. This allowed his team to create rich 3-D images of intramuscular fat and diseased arteries (J. Biophotonics, DOI: 10.1002/jbio.201100102).
Translational medicine researchers are evaluating Raman spectroscopy for a wide variety of purposes from interventional cardiology to home glucose monitoring. Some of those tools are quite mature, such as the Verisante Aura, a multimodal imaging and Raman spectroscopy instrument made by a government-funded Canadian company. In a study of 1,000 patients, it never missed a skin cancer lesion. Another business, C8 MediSensors, has developed a pocket-sized spectrometer that can monitor blood glucose levels through the skin. The company believes that it will earn regulatory approval to market the product in Europe next year.
Many researchers tried and failed to harness Raman spectroscopy and other infrared techniques to monitor blood sugar optically over the past several decades. Ramachandra R. Dasari, associate director of the George Harrison Spectroscopy Laboratory at Massachusetts Institute of Technology says those failures came down to an inherent drawback of Raman spectroscopy. It’s an inefficient process: Illuminate some tissue with a beam of monochromatic light, and only one in a million photons will undergo spontaneous Raman scattering. To get a good reading, you need an instrument that can collect many of those rare photons.
Using an optical component called a compound hyperbolic concentrator, Dasari’s team was able to build a portable Raman spectroscopy instrument that collects much more light than a conventional device. They used it to make noninvasive blood glucose measurements (AIP Advances, DOI: 10.1063/1.3646524). Recently they extended the work to measure several other metabolites.

None of those measurements would be possible without sophisticated calibration algorithms. The optical properties of tissue vary widely from person to person, and in any given person, they vary a lot from day to day. Dasari and colleagues Ishan Barman and Narahara Chari Dingari have developed sophisticated computer models that let them account for those sample-to-sample inconsistencies (Anal. Chem., DOI: 10.1021/ac101754n).
Other medical applications of Raman spectroscopy are just getting started, explains Eric O. Potma, a chemistry professor at UC Irvine. Potma and coworkers recently showed that they could use coherent anti-Stokes Raman spectroscopy to image cholesterol crystals within the blood vessels of rodents (J. Lipid Res., DOI: 10.1194/jlr.M018077). Cardiologists could use such a technique to examine the blood vessels of their patients, helping them gauge the severity of a vascular problem and personalize the treatments.
The slow march of optical spectroscopies toward the clinic has been helped along by technologies borrowed from communications electronics. Those technologies are allowing optics researchers to build things that would have been inconceivable or prohibitively expensive a decade ago. For example, Tromberg’s students were able to build a spatial frequency domain imaging system using an off-the-shelf digital light projector. In addition to using it to image their thesis adviser’s bicycling injuries, they have used it to measure the oxygenation of skin flaps during reconstructive breast surgery (J. Biomed. Opt., DOI: 10.1117/1.3614566). New light source and detector materials are allowing researchers to make smaller, cheaper devices. Some of those new instruments can operate at wavelengths that weren’t easily accessible until now.
Light projectors can also be used to superimpose multimodal images directly onto a patient’s body. A first glimpse of that can be seen in products like the AccuVein system, which uses infrared imaging to find veins. The same technology could be used to spot all sorts of analytes and then project a map of their location directly onto the body.
“If you’re an engineer working in optics, this is a very exciting market,” says Tom Hausken of Strategies Unlimited, a market research firm that is focused on the optics and photonics industry. His firm projects that the optical molecular imaging market will double between 2010 and 2014, reaching into the $400 million range. But that’s still very small compared with other industries, such as pharmaceuticals, Hausken points out.
Backers of optical medical diagnostics face a number of hurdles in getting their devices to market. Most notably, it’s not always easy to show that their devices are useful, user-friendly for doctors, or superior to widely used diagnostic techniques. That’s why the Beckman Laser Institute is acting as an incubator for four early-stage start-ups. By offering access to an active medical clinic, it may give those fledgling companies an opportunity to come out of the laser lab and into the limelight.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter