Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Homogeneous Glycoprotein Made
Carbohydrate Synthesis: Ten-year effort yields erythropoietin with sugars at native sites
by Stu Borman
October 8, 2012
| A version of this story appeared in
Volume 90, Issue 41

Thanks to a decadelong synthetic push, chemists finally have access to a homogeneous version of the blood-cell-producing glycoprotein erythropoietin (EPO). The product carries carbohydrates at the same sites as native EPO does. Native EPO is an inseparable mixture of proteins with variable carbohydrate modifications.
Glycoprotein synthesis specialist Richard Payne of the University of Sydney, in Australia, who was not involved in the new study, comments that “the robust methods developed and employed in this work will allow researchers to prepare a range of glycoproteins, including other EPO variants, to interrogate the role of glycosylation on biological activity and stability.”
Glycosylation’s influence on glycoprotein function is a black-box mystery that researchers and drug companies would love to better understand. More than $5 billion of commercial EPO, which is produced by engineered cells, was prescribed in the U.S. alone in 2011, according to health care industry information provider IMS Health.
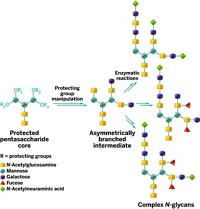
Samuel J. Danishefsky of Sloan-Kettering Institute for Cancer Research and Columbia University and coworkers have now created pure EPO with sugars at each of the four native glycosylation sites (Angew. Chem. Int. Ed., DOI: 10.1002/anie.201206090).
Danishefsky and colleagues used peptide synthesis to make four peptides, each containing one site of oligosaccharide attachment. To the one peptide with a native O-linkage glycosylation site, they linked a tetrasaccharide that occurs naturally in EPO. To each of the three peptides with native N-linkage sites, they attached a disaccharide. They then joined the four carbohydrate-decorated glycopeptides.
The product’s in vitro bioactivity approximates that of native EPO, Danishefsky says.
The three N-linked sugars in natural EPO are much more complex than the O-linked tetrasaccharide. Danishefsky is confident that his team will be able to complete the chemical synthesis of a more complex EPO soon.
The work follows related synthetic efforts by others. For example, groups led by Derek Macmillan of University College London and by Stephen B. H. Kent of the University of Chicago assembled native and mutant unglycosylated EPOs. In Japan, Yasuhiro Kajihara’s group at Osaka University made EPOs with an 11-unit sugar at a single native site. Some of those constructs have shown in vitro or in vivo bioactivity.
Macmillan calls Danishefsky’s synthetic effort “heroic” but adds that the product “falls considerably short of glycosylated EPO that would be produced biologically. So the total synthesis of EPO has not yet been achieved, but the report goes a long way and suggests that fully synthetic EPO is tangible, rather than pure fantasy.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter