Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Green Route To Glycols
Catalysis: Encapsulated complex drives epoxide-to-diol reactions without reagent excess
by Mitch Jacoby
October 22, 2012
| A version of this story appeared in
Volume 90, Issue 43
A metal-organic compound encapsulated in the nanosized pores of a crystalline solid selectively converts epoxides to diols in high yield under mild reaction conditions and with far less need for excess reagents, according to researchers at Dalian Institute of Chemical Physics, in China (Angew. Chem. Int. Ed., DOI: 10.1002/anie.201203774). The finding may help reduce costs and energy use in production of ethylene glycol and related products.
Ethylene glycol and other diols are key starting materials used to manufacture polyester resins, antifreeze, cosmetics, pharmaceuticals, and other products. Glycols are typically produced commercially via hydration of epoxides.
Ethylene glycol, for example, is made industrially through reaction of ethylene oxide and water, usually at elevated temperatures and in the presence of a 20-fold or larger excess of water. Those conditions drive high, selective conversion of ethylene oxide to ethylene glycol. But because of the large water excess, the product solution typically contains only 10 wt % ethylene glycol, which has a boiling point of 197 °C. The diol must be separated through energy-intensive distillation. Researchers have evaluated various alternative catalytic reactions, but they produce ethylene glycol nonselectively if the water-to-ethylene oxide ratio is less than 10.
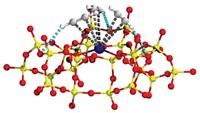
The Dalian team, which includes Bo Li, Qihua Yang, and Can Li, report that they have developed a catalyst that converts ethylene oxide to ethylene glycol readily at greater than 98% selectivity under mild conditions (40 °C) at a water-to-ethylene oxide ratio of less than two. They report similar results for several epoxides.
The team prepared the catalyst, a Co3+ complex with a salen ligand, by encapsulating the metal-organic compound in FDU-12, a nonporous silica material. They show that closely confining multiple catalyst molecules in the nanopores greatly enhances catalytic performance. The group narrowed the nanopore entrances via silylation reactions to prevent the catalyst from being leached from the silica by the reaction solution.
The work has “strong potential for industrial application,” says Zaiku Xie, director general of research at Sinopec, a major oil company in China. Because the catalyst is encapsulated in a solid, it is easily separated from the product and recycled, further enhancing the green and sustainable appeal of this work, he adds.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter