Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
A New Look At Harvesting Light
Biochemistry: Molecules key to color vision can be manipulated to alter their light-absorbing properties
by Celia Henry Arnaud
December 10, 2012
| A version of this story appeared in
Volume 90, Issue 50
\

\
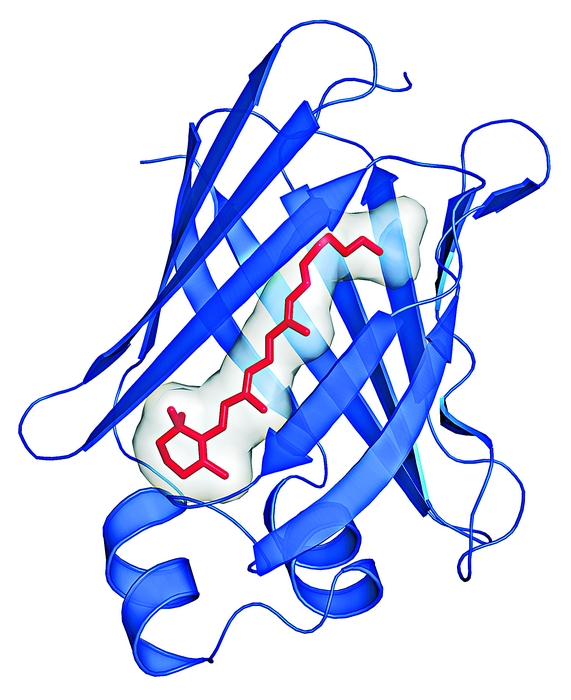
Retinal (red) bound to engineered proteins absorbs different colors, depending on the protein sequence. Cuvettes contain the same concentration of retinal but different proteins.
A better understanding of the molecules responsible for color vision—as well as a host of practical applications—may result from new chemical research on these light-absorbing chromophores.
Chemists at Michigan State University report that the protein environment can be manipulated to change a chromophore’s light absorption properties (Science, DOI: 10.1126/science.1226135). Ultimately, they say, the work could lead to new biobased sensors, light-harvesting proteins for solar energy, and new protein tags for biological research.
The pigmented rhodopsins, the light receptor proteins in color vision, all use the same molecule—11-cis-retinal—to absorb light across the visible spectrum. Nature has found a way to regulate 11-cis-retinal’s wavelength of maximum absorption in the human eye over a very large range, from about 420 to 560 nm, to detect various colors. Babak Borhan, James H. Geiger, and colleagues wanted to know what factors in the protein make such regulation possible. And they wanted to “red shift” the absorption profile as far as possible (toward longer wavelengths) to investigate the feasibility of expanding the visual palette.
To achieve their goals, they used cellular retinol-binding protein (CRBP) to mimic the electronic properties of rhodopsin-retinal complexes. They engineered CRBP so that it binds all-trans-retinal—a stable form of retinal that is easier to study than 11-cis-retinal—instead of retinol.
Engineering CRBP required two key mutations. First, they inserted a lysine in the right place to form an iminium bond with the retinal. “After we made that first mutation, that lysine was close to another lysine,” Borhan says, making the inserted one unreactive, so they replaced the second lysine with leucine. They then manipulated the electrostatic environment around the chromophore with other mutations. The resulting engineered proteins have absorption maxima (λmax) that range from 425 to 644 nm.
The researchers were surprised that their modifications pushed the absorption profile to wavelengths beyond values computational models had predicted to be possible. The most red-shifted protein, which has nine mutations, has a λmax of 644 nm, whereas previously no one thought retinal could have a λmax longer than about 620 nm, Borhan says.
“I think we have the opportunity to go even farther than 644 nm,” he says. “I’m not sure how much more we can go, but I don’t think we’re at the upper limit yet.”
In addition to having thus achieved a better understanding of the factors that lead to color vision, the Michigan State researchers plan to use the protein complexes as genetically encoded tags to label other proteins. “We have the ability to dial in whatever color we want, depending on the protein sequence,” Borhan says. They are also developing variants that bind fluorescent molecules. “Fluorescence is really the name of the game for protein fusion tags,” he says.
“We believe that our work provides a ‘toolbox’ of principles and strategies that can be used for controlling the spectroscopic properties of not just retinal, but potentially a variety of chromophores,” Geiger says.
The study “is a stunning example of design principles,” says Samie R. Jaffrey, a biologist at Weill Cornell Medical College. Borhan, Geiger, and coworkers have “figured out design principles for how you can rationally control the environment around a chromophore to change its absorbance properties.” He remains unsure, however, that the proteins will be practical protein tags.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter