Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biochemistry
A Better Disulfide Reducing Agent
Protein Biochemistry: Dithiobutylamine is a fast reducing agent for breaking cysteine-cysteine sulfur linkages
by Jeffrey M. Perkel
February 29, 2012

When biochemists want to break disulfide bonds within or between molecules, they add dithiothreitol to their buffer solutions. Now researchers describe an easy-to-make alternative to the small molecule that can reduce bonds more quickly. (J. Am. Chem. Soc., DOI: 10.1021/ja211931f).
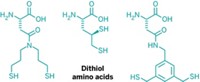
One of the main uses of dithiothreitol (DTT) is to keep proteins unfolded for accurate sizing on electrophoretic gels. But DTT could be more efficient, says Ronald Raines of the University of Wisconsin, Madison: Only deprotonated thiol groups can reduce cysteine-cysteine sulfur linkages, but at physiological pH, DTT’s thiol groups exist almost exclusively in the protonated form.
Raines and his team found a more efficient molecule called dithiobutylamine (DTBA). Like DTT, DTBA contains thiol groups at either end of a butane chain, but instead of DTT’s two hydroxyl groups, DTBA contains a single amine. The two compounds have comparable reducing potentials, but DTBA sports lower pKa values for its two thiols. As an added bonus DTBA, unlike DTT, is nearly odorless.
DTBA reduced the oxidized versions of the small molecules 2-mercaptoethanol and glutathione three to five times as fast as DTT could. It reduced the enzyme papain, which has a relatively anionic active site, 14 times as fast as DTT could, but reduced creatine kinase, with its more cationic active site, as efficiently as DTT did. DTBA is not yet commercially available, but researchers can prepare it from the cheap, abundant starting material
Raines points out that the amine in DTBA gives the molecule a “handle” that researchers could use to remove it from samples using ion exchange chromatography or functionalized beads. Alternatively, the amine could be coupled to other reagents, endowing those compounds with multiple functionalities. “There’s a lot of flexibility with this molecule,” he says.
CORRECTION: This story was updated on March 1, 2012, to clarify that making DTBA at industrial scale has been proposed by Ronald Raines.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter