Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Bright Lights, Single Cells
Cellular Analysis: A powerful source of infrared light reveals phosphorylation within individual cells
by Erika Gebel
April 11, 2012

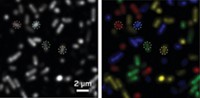
A bright light may help illuminate cellular secrets such as how they grow, differentiate, or respond to drug therapy. Researchers have focused infrared radiation from a synchrotron light source on single cells and measured protein modifications as the cells developed into nerve cells (Anal. Chem., DOI: 10.1021/ac300308x).
Phosphorylation—the attachment of a phosphate group to a protein—plays a key role in cells’ metabolism, proliferation, and differentiation. But the existing techniques for studying this process in single cells, including fluorescence-based ones, require researchers to put chemical tags on proteins of interest in advance. As a result, says Hoi-Ying Holman of Lawrence Berkeley National Laboratory, researchers can use the techniques only when they know what proteins they’re looking for.
IR spectroscopy can measure phosphorylation without labeling, but most instruments don’t use light bright enough to detect the weak signals from single cells. So Holman used a synchrotron, a source of intense IR radiation that generates data with a signal-to-noise ratio 100 to 1000 times as high as that of standard IR spectrometers.
To demonstrate the technique, Holman’s team used the synchrotron light to watch what happens inside individual rat cells called PC12 as they differentiated into nerve cells. Previous studies have shown that protein phosphorylation increases during this transition.
In the experiment, the researchers treated a culture of PC12 cells with a signaling protein called nerve growth factor, which triggers differentiation. Then, going one cell at a time, they focused a beam of light on a cell and collected data at several time points during the following hour and up to seven days later. Using data they collected previously from PC12 cells with artificially high levels of protein phosphorylation, the scientists could pinpoint the phosphorylation signal among the signals of other molecules in the cell.
Holman was surprised at how quickly phosphorylation levels changed in each of the cells. Levels spiked in just five minutes, she says, then came “in waves,” as the cells then experienced a decrease in phosphorylation, followed by a second rise. “It’s like you are watching a movie,” she says. The researchers observed that the timing correlates with the stage of differentiation, supporting phosphorylation’s role as a driving force in nerve cell maturation.
Studying phosphorylation in real time may be just the beginning. Since IR can track many types of molecules, Holman hopes to monitor other chemical transformations, such as methylation or changes in carbohydrate composition. She thinks it could prove useful to study cell signaling or the effects of radiation on cancer cells.
Likewise, Gregory Enns of Stanford University sees almost unlimited possibilities for the approach. “This is just great stuff,” he says. “The ability to interrogate a single cell is a wonderful tool with widespread implications.” Enns hopes to use this technique to test candidate drugs by comparing healthy cells to cells from people with diseases both before and after treatment.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter