Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Mass Spectrometric Technique Maps Biomolecules On Cell Surface
Cellular Imaging: Researchers measure mass of large biomolecules with high spatial resolution on single cells
by Alexander Hellemans
July 25, 2012

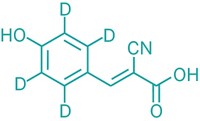
Using a new MALDI mass spectrometry method, researchers have mapped the distribution of two phospholipids on the surface of a cell with high spatial and mass resolution (Anal. Chem., DOI: 10.1021/ac301337h). This ability to spot specific biomolecules on a cell could find use in diagnosing disease, another scientist says.
Researchers want to map and analyze biomolecules, including large ones, on the surface of single cells to better understand physiological processes, such as how cells react to infections or how drugs affect diseases. To identify these molecules, researchers need a technique that can measure molecular mass with high precision.
The hurdle preventing researchers from applying high-resolution mass spectrometry to cells has been finding a workable ionization method. Some researchers have tried secondary ion mass spectrometry (SIMS), which uses a narrow ion beam to dislodge and ionize biomolecules from tissue or cells. The spatial resolution of SIMS is good enough to scan single cells, with a typical diameter of 10 µm. But the ion beam fragments the molecules, making it difficult to determine their original masses.
So other researchers have tried using a “softer” ionization source, such as the ultraviolet laser pulses used in MALDI. In this technique, researchers cover the cells with a matrix based on an aromatic acid to both protect the biomolecules from fragmentation and help them ionize. The pixel size of the resulting molecular mass map depends on the width of the laser beam.
In 2010, Bernhard Spengler and his colleagues at the Justus Liebig University Giessen, in Germany, reported mass spectrometry images of a human cancer cell using this “softer” approach with a pixel size of 2 µm (Rapid Commun. Mass Spectrom., DOI: 10.1002/rcm.4401). Although the spatial resolution was similar to that obtained with SIMS, the amounts of biomolecules that the laser ionized were too small to make analysis practical with current mass spectrometers.
So Spengler and coworker Andreas Römpp decided to use a wider laser beam to ionize more molecules. The result was MALDI images with somewhat larger pixels, 7 µm. To improve the precision of the data collected on the ionized biomolecules, they also used a new Fourier-Transform orbital-trapping mass spectrometer.
They obtained images of HeLa cells showing the spatial distribution of two phospholipids known as phosphatidylcholine PC(32:1) and PC(34:1) with resolution of a few micrometers. According to Römpp, obtaining such high spatial resolution of large molecules wouldn’t have been possible with SIMS.
“Before, you had to choose between spatial resolution and mass resolution,” he says, “and now you can get both in one measurement.”
Jeffrey Agar, an expert in developing matrix materials for MALDI at Brandeis University, says that instrument manufacturers should take note of Römpp and Spengler’s work because its spatial resolution beats commercially available instruments’, which can provide at best 25 µm.
And Kamila Chughtai, a researcher in the application of mass spectrometry imaging to cancer at the Foundation for Fundamental Research on Matter, in Amsterdam, is excited about extending this group’s work into a diagnostic tool. If MALDI works on tissue, she says, “It would allow us to see which cells are normal and which cells are cancer cells.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter