Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Nixing Nitric Oxide
Bioinorganic Chemistry: Reductase enzyme mimic provides insight on how pathogenic bacteria thwart NO attack
by Stephen K. Ritter
March 29, 2013
| A version of this story appeared in
Volume 91, Issue 13

Nitric oxide is a double-edged sword. It’s a key signaling molecule that controls blood pressure and nerve impulses, yet when concentrations of the simple gas molecule are too high, it’s toxic to cells. But this toxicity can be a valuable asset as well because it allows NO to serve as a central immune defense mechanism to destroy pathogenic bacteria such as Helicobacter pylori that invade the digestive system.
Some bacteria have countered by evolving to produce iron-based reductase enzymes that repel NO by reducing it to harmless nitrous oxide, N2O. Understanding how such enzymes work could lead to new drugs to thwart the bacterial NO defense mechanism and prevent infections.
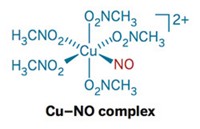
A team of chemists at the University of Michigan has now taken a step toward this goal by developing a diiron complex that serves as the first functional model for the catalytic site of an NO reductase enzyme (J. Am. Chem. Soc., DOI: 10.1021/ja309782m).
Nicolai Lehnert and coworkers determined how the diiron complex bearing a nonheme ligand system with an additional flavin cofactor binds two molecules of NO and, in a two-electron reduction process, converts them to N2O and water. The Michigan system is significantly faster and more efficient in generating N2O than other reductase models.
Besides new drugs, the mechanistic insight from studying the diiron complex could be important in nonbiological applications, Lehnert notes, such as the use of electrocatalysts to decompose nitrogen oxide pollutants in automobile and power plant exhaust.
“There is a growing interest in flavodiiron NO reductases owing to their possible roles in the detoxification of NO,” notes Peter C. Ford, whose group at the University of California, Santa Barbara, studies NO bioactivity. “Lehnert and coworkers have provided a very interesting diiron functional model,” he says, “that certainly adds to the growing recognition of the importance of nonheme iron complexes in the chemical biology of NO.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter