Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Bringing Ancient Proteins To Life
Scientists resurrect long-gone cellular workhorses to study evolution and engineer new versions of proteins
by Celia Henry Arnaud
April 8, 2013
| A version of this story appeared in
Volume 91, Issue 14
\
\
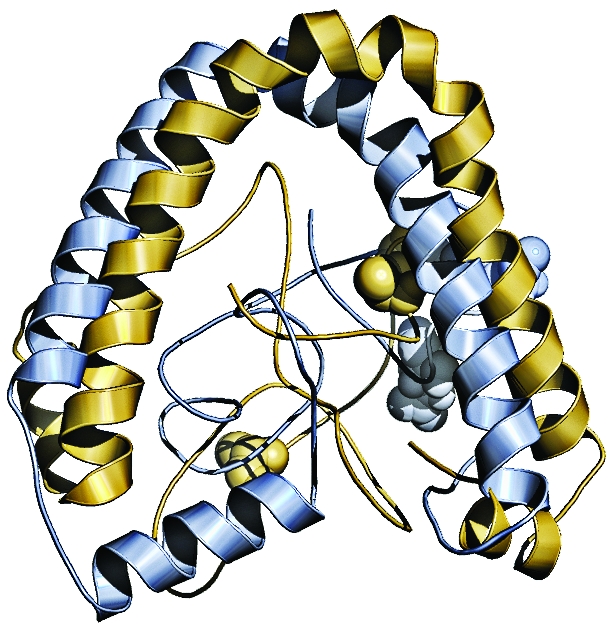
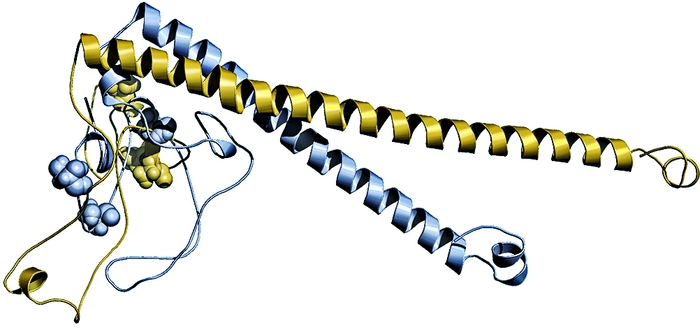
A change of three amino acids caused these two ancestors (shown overlaid on each other) of the modern transcription factor CEBPB to respond differently to phosphorylation. In the inactive kinked conformation (top), the older ancestor protein (blue) is phosphorylated, but the newer ancestor protein (yellow) is unphosphorylated. In the active extended conformation, the reverse is true. The phosphorylation sites are shown as spheres.
Ancient proteins differed from their modern descendants in sequence and, sometimes, function. Scientists would like to know how those proteins actually worked. Unfortunately, they disappeared with the organisms that used them, and scientists can’t time travel to study them.
Or can they? When it comes to studying ancient proteins, scientists have their own version of time travel. By comparing the sequences of closely related modern proteins, scientists can deduce the sequence that appeared in their common ancestors. They can then synthesize those ancestral proteins in the lab, using either bacteria or in vitro systems to resurrect them.
Scientists use those resurrected proteins in multiple ways. Evolutionary biologists use them to understand evolutionary history and how proteins acquire new functions. Protein engineers use them as starting points for designing proteins with new functions.
By using the sequences of closely related proteins from multiple species, scientists can build a so-called phylogenetic tree that graphically depicts the relationships between the proteins. The tip of each tree branch represents a modern protein. Moving backward down the branch, each point represents a different ancestor of that modern protein. When a branch splits from a neighboring branch, the protein at the bifurcation point, or node, is the last common ancestor of proteins at the tips.
“We have algorithms to infer computationally the sequence at every node in the tree,” says Eric A. Gaucher, an evolutionary biologist at Georgia Institute of Technology. “After we get the output from the computational analysis, we physically build those ancestral genes in the laboratory. We will use a modern organism or even an in vitro protein translation system to make the ancestral proteins. Then we measure properties of those proteins.”
This type of study was first carried out in the mid-1980s by Steven A. Benner, then at Harvard University, now at the Foundation for Applied Molecular Evolution.
Resurrecting proteins from bygone eras can help put the inferences made by evolutionary biologists on firmer molecular footing. “Evolution involves statements and explanations that have a historical component. One problem with historical explanations is that, absent time travel, you have no good way of doing experimental tests,” Benner says. But with resurrected proteins, scientists can test evolutionary hypotheses.
Günter P. Wagner, an evolutionary biologist at Yale University, uses resurrected proteins to study how pregnancy evolved. For example, three amino acid substitutions in the ancient version of a transcription factor called CEBPB from the last common ancestor of marsupials and placental mammals, which lived 175 million years ago, transformed it from a repressor into an activator. It now activates production of the pregnancy hormone prolactin. The changed version, which was produced by the last common ancestor of placental mammals 105 million years ago, eliminated two phosphorylation sites and introduced a new one, altering the protein’s response to a signaling molecule (Nature, DOI: 10.1038/nature10595).
In that case, Wagner was able to determine the chemical changes that led to the mechanistic changes. In other cases, so many amino acids change that researchers have not yet been able to identify those responsible for evolution of the proteins. But Wagner is not discouraged.
\

\
By the time the Chinese were using this now-4,000-year-old wine vessel, humans and their primate ancestors had long expressed an enzyme capable of metabolizing ethanol.
“This approach and similar approaches where we can do laboratory experiments with ancestral proteins are essential to really nail down whether what we think about the past was mechanistically possible or plausible,” Wagner says.
Benner also continues to work with ancestral proteins. He recently used protein evolution techniques to answer the question of when the primate ancestors of humans were first able to metabolize ethanol. The yeast fermentation pathway responsible for producing ethanol arose about 80 million years ago, but when did primates, which arose about 60 million years ago, acquire the ability to metabolize ethanol? Some evolutionary biologists have posited that ethanol metabolism is a recent development, which might explain alcoholism and other alcohol-related diseases as products of inadequate evolution in response to a recent change.
Using resurrected ancestors of primate alcohol dehydrogenases, Benner showed that the enzymes first acquired the ability to metabolize ethanol about 10 million years ago, which was before the human ancestral line diverged from gorillas and chimpanzees (PLoS One, DOI:10.1371/journal.pone.0041175). Thus, human ancestors were first exposed to alcohol long before the beginnings of agriculture roughly 10,000 years ago.
In another study, Gaucher, working with scientists at the University of Granada, in Spain, resurrected ancestors of modern β-lactamases, microbial enzymes that disable β-lactam antibiotics such as penicillin and cephalosporins (J. Am. Chem. Soc., DOI: 10.1021/ja311630a). Modern β-lactamases tend to prefer specific substrates. The team wanted to know whether ancient β-lactamases were just as choosy.
The enzymatically promiscuous ancestral form could bind multiple substrates, Gaucher says. However, “it couldn’t act on those substrates very well,” he says, “and over time there was selective pressure to be able to hydrolyze one substrate very well.” Its descendants therefore became specialists.
Dan S. Tawfik of the Weizmann Institute of Science, in Rehovot, Israel, outlines some inherent limitations of ancestral reconstructions with respect to the evolutionary history of enzyme families. “To predict an ancestor, you need to align sequences with 100% reassurance,” he says. Most ambiguity in determining ancient sequences involves regions where changes in length or significant structural rearrangements occurred as modern proteins evolved, and it is such regions that often underlie key historical functional changes, he says.
But answering questions about evolutionary biology is only part of what can be achieved with resurrected proteins. Tawfik is finding ancestral reconstruction useful in protein engineering as well. For instance, his group has started using resurrected ancestral proteins to design libraries because ancient sequences can suggest worthwhile variations.
“Ancestral proteins tend to be a lot more stable than modern proteins,” Gaucher says. “They’re resistant to high temperatures, extreme pH, and slightly deleterious mutations. Because the ancestral proteins have these really robust properties, they serve as great starting points for directed-evolution experiments. You can subject them to more extreme conditions and a lot more mutations. The more mutations you have, the more likely you are to come up with a novel function.”
Protein evolution techniques can also be used to find enzymes that can catalyze new reactions. Romas J. Kazlauskas, a biochemist at the University of Minnesota, Twin Cities, usually searches for a new enzyme by starting with an existing enzyme with a mechanism or properties close to those desired. Then he uses protein engineering to “fix it.” But modern proteins are often so specialized that they’ve backed themselves into a corner that protein engineering can’t get them out of.
Kazlauskas and evolutionary biologist Antony M. Dean, also at Minnesota, are now resurrecting ancient enzymes in the hope that those proteins might be more “generalists” than modern “specialist” enzymes.

“Our idea is if we go back and re-create some of these generalists, they might be catalytically promiscuous—they may catalyze several reactions, including the one that we want,” Kazlauskas says. “Starting from those enzymes, we might have an easier time evolving new enzymes.”
With that goal in mind, Kazlauskas and Dean have been resurrecting common ancestors of hydroxynitrile lyases and esterases. The hope is that the ancestors will have both forms of activity and thus be better able to evolve new functions. The ancestors they’ve resurrected are indeed functionally promiscuous and catalyze reactions at higher rates than their modern counterparts.
Biologists would like to move beyond resurrecting one or two proteins at a time to resurrecting whole pathways or systems. “Your body works with dozens and dozens of proteins,” Benner says. “When we resurrect the ancient alcohol dehydrogenase, that enzyme is converting ethanol to acetaldehyde. We should also resurrect the ancient acetaldehyde-oxidizing system, which makes acetate, and we should resurrect the ancient acetate-oxidizing system.”
But why stop at just individual pathways? “We’d like to resurrect a complete genome for a microbe,” Gaucher says. “We’d like to synthesize an ancestral genome and jump-start it in a modern organism.” Some similar efforts are already under way (see page 34).
In 2010, Gaucher started the company General Genomics, in Atlanta, to bring the power of ancestral resurrections to the industrial arena. The company, which received its first round of investor financing last November, focuses on biomedical and agricultural applications.
Researchers in the protein evolution field hope the approach will continue to evolve and grow, but it won’t be easy. “You have to be reasonably fluent in molecular biology and evolutionary biology to really take advantage of the method,” Wagner says. “There are few people who challenge themselves to do both.”




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter