Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Making Amines And Alcohols Enantioselectively
ACS Meeting News: Simple organic molecules catalyze reaction of unsaturated organoboron reagents with imines and carbonyls
by Bethany Halford
April 15, 2013
| A version of this story appeared in
Volume 91, Issue 15
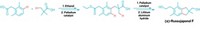
Chiral amine and alcohol functional groups dominate the landscape of biologically relevant molecules, such as drugs and agrochemicals. So chemists are always on the lookout for ways to make such moieties. Chemists at Boston College, led by Amir H. Hoveyda and his team, which includes graduate student Daniel L. Silverio, have come up with a simple way to synthesize amines and alcohols enantioselectively (Nature, DOI: 10.1038/nature11844). The reaction adds an allyl group, from an allylboronic ester, to an activated imine (shown) or to an electron-deficient carbonyl. The key component in the transformation is an organic catalyst that orchestrates the enantioselective carbon-carbon bond-forming reaction. The researchers prepare the catalyst from the abundant amino acid valine in just four steps through the use of inexpensive reagents. As little as 0.25 mol% is all that’s needed of the catalyst to produce allylamines and allyl alcohols in more than 85% yield with high enantioselectivity. Furthermore, the reaction takes place in common solvents at room temperature in less than six hours. The team now aims to use this catalyst system to develop other efficient and enantioselective C–C bond-forming reactions.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter