The images appearing on the computer screen were almost too detailed and fast-moving to take in, Misha B. Ahrens remembers. He and colleague Philipp J. Keller were recording the activity of about 80,000 neurons in a live zebrafish brain, the first time something on this scale had been done. Cross-sectional pictures of the young fish’s head flew by, dotted with splotches of light.
Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Mapping The Brain Onto The Mind
BRAIN initiative aims to improve tools for studying neurons to answer questions about human thought and behavior
by Lauren K. Wolf
April 21, 2013
| A version of this story appeared in
Volume 91, Issue 16
The Howard Hughes Medical Institute (HHMI) neuroscientists were using a zebrafish larva with a fluorescent protein inserted in its neurons, and the protein was lighting up every time the cells fired. Their custom-built microscope imaged and recorded the resulting lightning storm in the fish’s brain in real time.
NEUROTECHNOLOGY TOOL KIT
The BRAIN initiative will likely build on available techniques for recording and manipulating neuron dynamics.

Credit: Courtesy of Ed Boyden
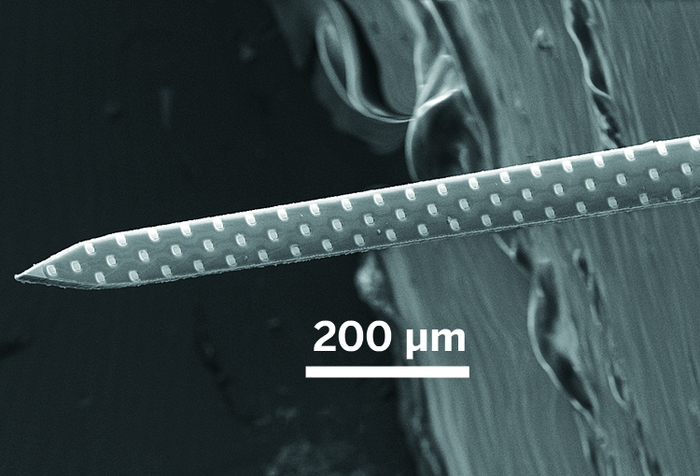
Credit: Courtesy of Sotiris Masmanidis
Electrical Probing: Neuroscientists plunge microelectrode arrays, like the 64-channel version depicted here, into the brains of model organisms to measure voltage from nearby neurons.

Credit: Travis Nelson/Inscopix
Imaging: Researchers use fluorescent calcium indicators and advanced optics, like the microscope shown here for mounting on a mouse’s head, to visualize nerve cell firing in animals’ brains.
Ahrens commemorated the milestone experiment—which took place nearly seven months ago in a lab at the institute’s Janelia Farm Research Campus outside Washington, D.C.—by filming it with his iPhone. “It was mind-blowing to see the entire brain flash past our eyes,” he remembers.
Keller sat in awe at the computer, repeatedly pulling up and admiring slices of data the high-speed apparatus was collecting. The translucent zebrafish, immobilized in a glass tube filled with gel and nestled among the microscope’s optics, was completely unaware that its neural processing was causing such a stir.
Up until that point, scientists had been able to record simultaneous activity from only about 2 to 3% of the 100,000 neurons in a young zebrafish’s head, Keller says. He and Ahrens managed to capture 80%—a giant leap for fishkind.
On March 18, the duo reported their brain-imaging feat online at Nature Methods (DOI: 10.1038/nmeth.2434). Just 15 days later, President Barack Obama announced a large-scale neuroscience initiative to study the dynamics of brain circuits (C&EN, April 8, page 9).
Unlike the Human Connectome Project—a federal program that strives to uncover a static map of the brain’s circuits—this new initiative aims to uncover those circuits’ activity and interplay. BRAIN (Brain Research through Advancing Innovative Neurotechnologies), as the project is called, will get $100 million in federal support if Obama’s request is granted (see page 25), and it will get a similar amount from private foundations such as HHMI in 2014.
“It was a coincidence,” Keller says of the timing of the proposal. He and Ahrens weren’t involved in developing BRAIN, but their goal—to record all the activity from all the neurons in a simple organism’s brain at once—falls directly in line with the initiative.
Eighty-thousand neurons is a lot. But it’s nothing compared with the 85 billion nerve cells that humans have in their brains, or even the 75 million that mice have. To make the leap to measuring large swaths of the brain circuits of rodents or even humans, BRAIN researchers will need to develop new methods of measuring neuronal activity. They are already working on molecular tags to more accurately indicate nerve cell firing in real time. And scientists are developing miniaturized probes to monitor brain cells without disturbing the organ itself, as well as faster techniques for analyzing the flood of data generated by such a huge number of neurons.
Some imaging methods that monitor multitudes of neurons, like that of Ahrens and Keller, already exist. As do techniques for probing scads of nerve cells with tiny electrodes. BRAIN will likely build on these technologies, experts say. But it will also shoot to build “dream” technologies such as implantable nanomaterials that transmit the activity of individual neurons from inside the head.
At the moment, however, no one knows the exact scope of BRAIN. The National Institutes of Health has already appointed a team of neuroscientists to draw up a blueprint for what should be a multiyear initiative. Other federal agencies involved—the National Science Foundation and the Defense Advanced Research Projects Agency—have yet to announce their strategies.
“Neuroscience is getting to the point where researchers cannot take the next big step to understand neural circuits armed with traditional technology,” says Rafael Yuste, a neuron-imaging expert at Columbia University.
And taking that step, he argues, is vital to understanding human thought. “We have a suspicion that the brain is an emerging system,” Yuste says. In other words, how the brain produces memories or actions involves the interactions of all its neurons, rather than just one or even 1,000. It’s like watching television, Yuste adds. “You need to see all the pixels, or at least most of them, to figure out what’s playing.”
Along with five other scientists, Yuste made the original pitch for a public-private project to map the brain’s dynamics in a 2012 article in Neuron (DOI: 10.1016/j.neuron.2012.06.006). The group argued that not only could this approach help reveal how the human mind works, but it might also offer some insight into what happens when the brain malfunctions. Knowing how the brain’s circuits are supposed to function, Yuste says, could help pinpoint what’s going wrong in conditions such as schizophrenia, which likely involve faulty circuitry.
BRAIN proponents also say areas outside of science and medicine could profit from the initiative. If successful, they claim, BRAIN could yield economic benefits similar to the Human Genome Project, a program launched in 1990 to sequence all the base pairs in a person’s DNA. “Every dollar we spent to map the human genome has returned $140 to our economy,” President Obama noted when he announced BRAIN.
AS WAS THE CASE for the Human Genome Project, BRAIN has been criticized by many scientists. In an already-tight fiscal climate, some researchers have voiced worries that paying for the initiative will mean losing their own funds. And others have expressed reservations that the project is going after too many neurons to yield interpretable, useful results.
But no one seems to dispute that better tools to record activity from nerve cells is a worthwhile goal. “There’s definitely room to grow in many of the techniques we use to record brain activity,” says Mark J. Schnitzer , a neuroscientist at Stanford University. So far, he says, progress has been made mainly by individual labs doing their own thing. But to get to the next level more rapidly, a coordinated effort like BRAIN—centers and labs of neuroscientists, chemists, and researchers in other disciplines working together— might be the ticket.
Until recently, the number of neurons being recorded simultaneously in experiments was doubling every seven years, according to a 2011 review in Nature Neuroscience (DOI: 10.1038/nn.2731 ). But the Janelia team blew this trend out of the water with its high-speed camera and microscope, which rapidly illuminates and images slices of the brain.
The Janelia experiment worked primarily because zebrafish larvae are transparent to light and can be easily immobilized without negative consequences to their brain activity. But moving to mice, which have more neurons and a light-impenetrable skull, will require some more serious innovation, Keller adds.
Some researchers have designed implantable prisms and fiber-optic probes to direct light into the depths of the mouse brain. But those optical tricks are still limited to measuring a few hundred neurons at once. Plus, the mouse has to be tethered to the fibers or prevented from moving altogether.
Stanford’s Schnitzer has overcome the mobility issue with a miniaturized microscope that he and his team designed to fit onto a mouse’s head. Standing three-quarters of an inch tall, the lightweight device, which contains its own light source and camera, gets implanted into the rodent’s brain, enabling researchers to track the freely moving animal’s nerve cell activity.
Early this year, Schnitzer’s group used the setup to follow the dynamics of roughly 1,000 neurons in a mouse’s brain for more than a month (Nat. Neurosci., DOI: 10.1038/nn.3329). The team learned that neurons in one part of the mouse’s brain fired in similar patterns whenever the mouse returned to a familiar spot in its enclosure.
Still, such optical techniques are invasive. “The most elegant experiment would be done from the outside, without mechanical disturbance to the brain,” Columbia’s Yuste says. He’d like to see BRAIN help develop new light sources that can penetrate farther into brain tissue than a few millimeters.
Also on Yuste’s neuron-imaging wish list is a better way to indicate cell firing. As in the Janelia experiment and Schnitzer’s microscope study, the imaging of neuronal activity is typically carried out with calcium indicators. These are molecules that move to the insides of neurons or are proteins engineered to reside there, both designed to fluoresce when they bind to calcium ions.
As a nerve cell fires, its ion channels open, allowing calcium ions to trickle inside and trigger the indicators.
However, “calcium imaging is flawed,” Yuste says. “It’s an indirect method of tracking neuronal firing.” The indicators can’t tell scientists whether a nerve cell fired a little or a lot, he argues. And they don’t track the cells’ electrical activity in real time because calcium diffusion and binding are comparatively slow.
So Yuste and others are working to develop dyes or nanomaterials, called voltage indicators, that bind within a neuron’s membrane and optically signal the cell’s electrical status. Progress is slow-going, however, because a cell’s membrane can hold only so many indicators on its surface and the resulting signal is low.
Another way neuroscientists are more directly measuring nerve cells’ electrical activity is with miniaturized electrodes and nanowires. These probes measure, at submillisecond speeds, the electrical current emitted by a neuron when it fires.
“But anytime you plunge anything into the brain, you have to worry about tissue damage,” says Sotiris Masmanidis, a neurobiologist at the University of California, Los Angeles. “The concern is, how much are you perturbing the system you’re studying?”
To minimize tissue disturbance, Masmanidis and others are lithographically fabricating arrays of microelectrodes that can record nerve cells’ electrical signals from 50 to 100 µm away. So far, the UCLA researcher says, electrode arrays are capable of measuring, at most, 100 to 1,000 neurons at a time.
Determining what types of nerve cells an arrayed microelectrode is measuring, however, is not exactly straightforward, given that it blindly measures any neuron in its vicinity, Masmanidis says. To figure it out, scientists have to take extra steps and monitor the cells’ reaction to drugs or other modulators.
But what good is measuring the dynamics of a slew of nerve cells without having any idea why they’re firing? BRAIN supporters think one way of getting an answer to which environmental cues or perceptions trigger certain neuronal activity patterns is a technique called optogenetics.
Hailed by Nature Methods as the “method of the year” in 2010, optogenetics enables scientists to activate particular nerve cells in the brains of animals with light. The researchers first engineer light-activated proteins into a mouse’s neurons and then trigger the macromolecules via fiber-optic arrays implanted in the rodent’s brain.
Once researchers have measured a firing pattern from an animal’s nerve cells, they can later play it back to see what happens, says Edward S. Boyden, an optogenetics pioneer and neurobiologist at Massachusetts Institute of Technology. “Once we ‘dial’ an activity pattern into the brain,” he says, “if we see that it’s enough to drive some behavior, that could be quite powerful for understanding which parts of the brain drive specific functions.”
Researchers have already been optogenetically stimulating clusters of a few hundred cells in mice, investigating the rodents’ decision-making abilities and aggressive tendencies.
But a brain is more than just electrical activity, says Anne M. Andrews, a psychiatry professor at UCLA. It also uses at least 100 types of neurotransmitters that are involved in triggering neuronal activity at cell junctions, or synapses. “If we want to understand how information is encoded in neuronal signaling, we have to study chemical neurotransmission at the level of synapses,” Andrews says.
And what better way to do that than with nanotechnology? asks Paul S. Weiss, a chemist and nanoscience expert, also at UCLA. After all, the junctions between neurons are just 10 nm wide, he adds.
Andrews and Weiss are hoping BRAIN will support the development of nanoscale sensors to measure the chemical activity at synapses. And they’re already in talks with UCLA’s Masmanidis to functionalize channels on his microelectrodes with molecules that could sense neurotransmitters.
No matter what BRAIN ends up encompassing, one thing is clear: Advances in the numbers of neurons monitored will necessitate improvements in data analysis and storage.
Take, for instance, the experiment done at Janelia. That single session of recording from a zebrafish brain generated 1 terabyte of data. “So you can fit two or three experiments on a computer hard drive,” Ahrens says. “It’s not a bottleneck yet, but when we start creating faster microscopes, computational power might become a problem.”
He and Keller also have just scratched the surface when it comes to analyzing the data they obtained from their initial experiments. As they reported in their Nature Methods paper, the pair found a circuit in the fish’s hindbrain functionally coupled to a specific part of its spinal cord. But determining what that means and what the rest of the brain is doing will require more study and help from computational neuroscientists.
“It’s apparent that to really understand what the brain is doing, you need to have as complete information as you can,” Ahrens says. “It’s a good goal to have, to measure as many neurons as possible.” But it’s a challenging one.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter