Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Deciphering Ordered But Noncrystalline Structures
Method combines X-ray diffraction, NMR, and modeling to pick out structures that are tough to determine
by Jyllian Kemsley
April 29, 2013
| A version of this story appeared in
Volume 91, Issue 17
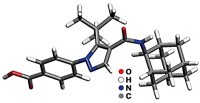
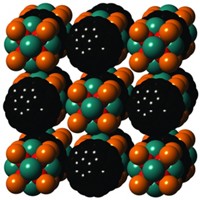
Zeolites and layered silicate clay materials have a variety of applications, including catalysis and chemical separations. Although the materials are molecularly ordered, pinning down their exact structures can be difficult because they may not form crystals large enough for single-crystal X-ray diffraction. A new approach to determining structures of such solids combines powder X-ray diffraction, 29Si nuclear magnetic resonance spectroscopy, and computer modeling (J. Am. Chem. Soc., DOI: 10.1021/ja311649m). A research team led by Bradley F. Chmelka of the University of California, Santa Barbara, and Sylvian Cadars of France’s National Center for Scientific Research demonstrated the approach on a nanocomposite clay made of silicate layers separated by an alkylammonium surfactant. The researchers obtained data on repeating structural units from the X-ray analysis and on silicon bonding from NMR. They used those data to narrow the number of possible framework structures. With density functional theory, they were able to identify two structures that best represent the nanocomposite.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter